Why Does Volume Increase When Temperature Increases
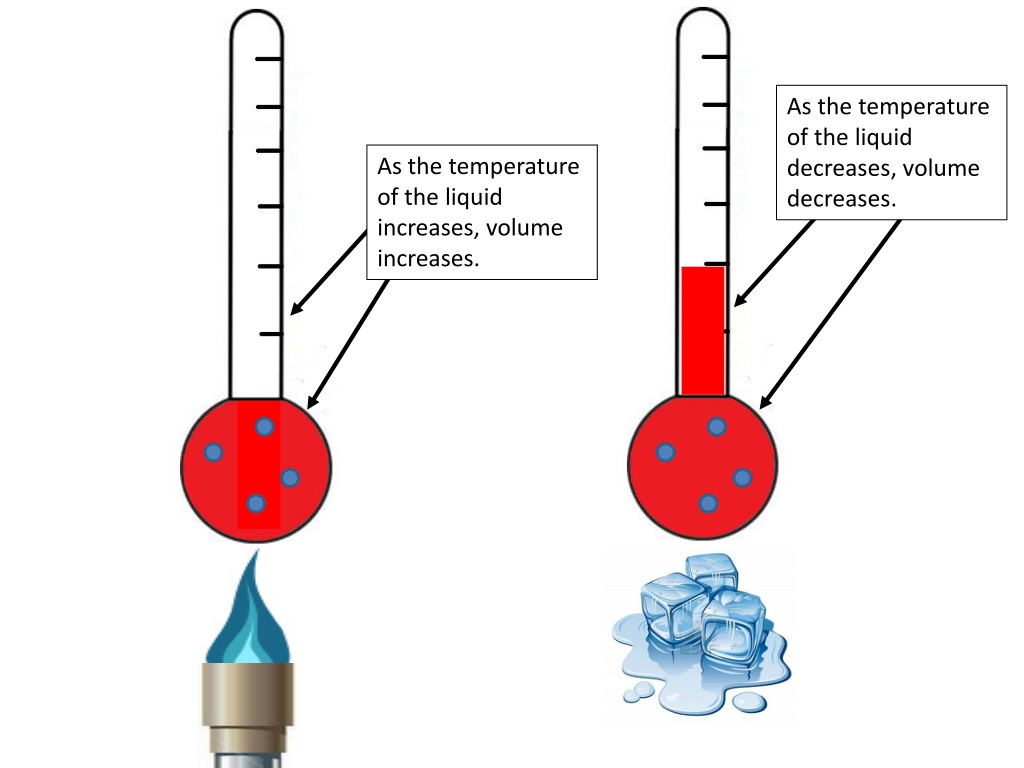
Ever feel like your apartment gets a little… snugger on a hot day? Or maybe you’ve noticed your tires look a bit flatter after a cold snap? There’s a perfectly good scientific reason for that, and it’s all about how temperature and volume are besties. Think of it like this: when things heat up, they tend to loosen up, expand, and take up more space. It’s a little like us after a really satisfying meal – we just need a bit more room!
This isn't some abstract concept confined to fancy laboratories with bubbling beakers and lab coats. Nope, this is happening all around you, all the time, influencing everything from the way your comfy couch feels to how the very air you breathe behaves. So, let’s dive into this delightful dance of heat and expansion, shall we? It’s less about complex equations and more about understanding the energetic hum of the universe.
The Energetic Hustle of Molecules
At the heart of this phenomenon are tiny, invisible particles – the building blocks of everything: molecules. Whether it’s the air in your room, the water in your tea, or the metal in your car keys, it’s all made of these energetic little guys. And just like us, when they get a shot of energy, they get… well, a bit more active.
Imagine them as tiny dancers on a dance floor. At cooler temperatures, they’re shuffling along, doing a polite waltz. But when the temperature cranks up, it’s like the DJ drops a killer beat! They start to jig, jump, and spin with way more gusto. This increased movement, this kinetic energy, means they need more space to do their happy dance.
They bump into each other more, they push each other further apart, and the overall effect is that the substance they make up starts to expand. It’s a bit like a crowded mosh pit at a concert – when the music gets louder and faster, everyone starts jostling and taking up more space. The more intense the music (heat), the bigger the mosh pit (volume).
Gases: The Ultimate Expansion Artists
Gases are the rockstars of this expansion show. Because their molecules are already pretty far apart and zipping around freely, a small increase in temperature can lead to a noticeable increase in volume. Think about a hot air balloon. When the air inside is heated, it becomes less dense than the surrounding cooler air. This difference in density, driven by the expansion of the hot air, is what lifts the balloon into the sky.
This is also why you should never, ever throw an aerosol can into a fire. That can is packed with gases under pressure. Heat it up, and those gases will expand with incredible force, potentially leading to a rather dramatic (and dangerous) explosion. So, a quick tip: if you’ve got hairspray or canned air, keep it away from direct heat sources. Your eyebrows will thank you.
It’s also a principle behind some nifty everyday technologies. Ever seen a thermometer with mercury or alcohol? As the liquid inside heats up, it expands and climbs up the narrow tube, indicating the temperature. Simple, elegant, and all thanks to those bustling molecules!
Liquids: A More Reserved Expansion
Liquids also expand when heated, but they’re a bit more reserved about it. Their molecules are closer together than in gases, and they have a bit more of a reluctant clinginess. So, while they’ll loosen up and spread out, it’s not quite the wild free-for-all you see with gases. Still, the effect is significant enough to be important.
Consider the expansion of water. If you fill a pot right to the brim with cold water and then heat it, you’ll notice the water level creeps up. This is why it’s important to leave a little headspace when cooking or baking with liquids, especially if you’re using a double boiler or simmering something for a while. Nobody wants a messy kitchen counter that looks like a failed culinary experiment.
This expansion is also crucial for engineers. When designing bridges, for instance, they have to account for the expansion and contraction of materials due to temperature changes. You might have seen those gaps in bridges – they're not mistakes! They're carefully designed spaces that allow the bridge to expand and contract without buckling or breaking. Pretty cool, right? It’s like giving the bridge a little bit of breathing room.
Solids: The Stoic Expanders
Solids are the most stoic of the bunch when it comes to expansion. Their molecules are packed tightly together, vibrating in fixed positions. Even so, when you heat them, those vibrations get a little more vigorous, and the molecules push against their neighbors just enough to cause a slight overall expansion. It’s like a very polite crowd shuffling forward a tiny bit when someone offers them a free cookie.
This is why power lines sometimes sag in the summer. The metal wire heats up, expands, and gets longer, causing it to droop. In the winter, it contracts and becomes taut again. It’s a constant ebb and flow dictated by the weather. You’ve probably seen those telephone poles with those zig-zaggy things on the wires – those are actually insulators designed to allow for this expansion and contraction without stressing the wire or the poles.
This property is also fundamental to how thermometers work (beyond liquid thermometers). Think about bimetallic strips, often found in older thermostats or oven thermometers. These are made of two different metals with different expansion rates bonded together. When heated, one metal expands more than the other, causing the strip to bend, which then moves a pointer to indicate the temperature. It’s a clever piece of engineering driven by the fundamental behavior of matter.
Why This Happens: The Underlying Science (No Math Required!)
So, why does this all happen? It boils down to energy. Temperature is essentially a measure of the average kinetic energy of the particles in a substance. When you add heat, you’re adding energy. This energy makes the particles move faster and vibrate more intensely.
These faster-moving particles need more space to bump and jostle around. They push against their neighbors, and the collective effect is an increase in the overall volume of the substance. It’s like giving your party guests a bigger dance floor – they can really cut loose!
Think of it as a chain reaction. One molecule gets more energy, moves more, bumps into its neighbor, giving that neighbor more energy, and so on. This cascading effect ripples through the entire substance, leading to expansion. It’s a beautiful, natural phenomenon that demonstrates the interconnectedness of everything at a molecular level.
Practical Tips for Everyday Life
Understanding this concept isn't just for science nerds; it has practical applications that can make your life a little smoother:
- Car Tires: Check your tire pressure when the tires are cold. As you drive, the tires heat up, and the air inside expands, increasing the pressure. This is why you might find your tires a bit overinflated after a long drive on a hot day. For optimal safety and fuel efficiency, it's best to adjust pressure when tires are cool.
- Cooking: As mentioned, leave a little room when heating liquids. Don't fill your pots to the brim, especially if you're simmering something. This prevents messy boil-overs.
- Sealed Jars: Ever struggled to open a tight jar lid? Running the lid under hot water can help! The metal lid heats up and expands slightly, loosening its grip on the glass jar. It’s a little trick that’s as old as time.
- Home Heating: Radiators and heating pipes are designed with expansion in mind. If you hear creaking or pinging sounds from your heating system, it might just be the metal expanding as it heats up.
- Gardening: When planting seeds or small plants, give them some space. As they grow, they’ll take up more room, and this natural expansion is a sign of healthy growth.
Cultural Tidbits and Fun Facts
This phenomenon isn't just a modern scientific discovery. Ancient civilizations were aware of the practical implications of thermal expansion, even if they didn't have the molecular explanations. The Romans, for instance, understood how metals expanded and contracted, which was crucial for their engineering marvels like aqueducts and the Colosseum.
Did you know that the world’s longest railway bridge, the Lake Pontchartrain Causeway in Louisiana, has expansion joints to accommodate the thermal expansion and contraction of its concrete structure? It’s a testament to how this basic principle impacts even the grandest of human constructions.
And here’s a fun one: the iconic Golden Gate Bridge in San Francisco actually gets about 10 feet taller in the summer than in the winter due to thermal expansion! Imagine that – a giant metal structure breathing with the seasons.
A Gentle Reflection
So, the next time you feel a little warmer, or notice something expanding, take a moment to appreciate the quiet, energetic dance happening at the molecular level. It’s a constant, fundamental process that shapes our world, from the grandest mountains to the smallest droplets of dew.

It’s a reminder that everything is in flux, constantly responding to its environment. Just like us, things loosen up, grow, and adapt when they’re given a little warmth and energy. There’s a certain beauty in that gentle, persistent expansion, a sign of life and change in the material world. It’s a subtle, everyday miracle, and understanding it just adds another layer of wonder to our already fascinating existence.
