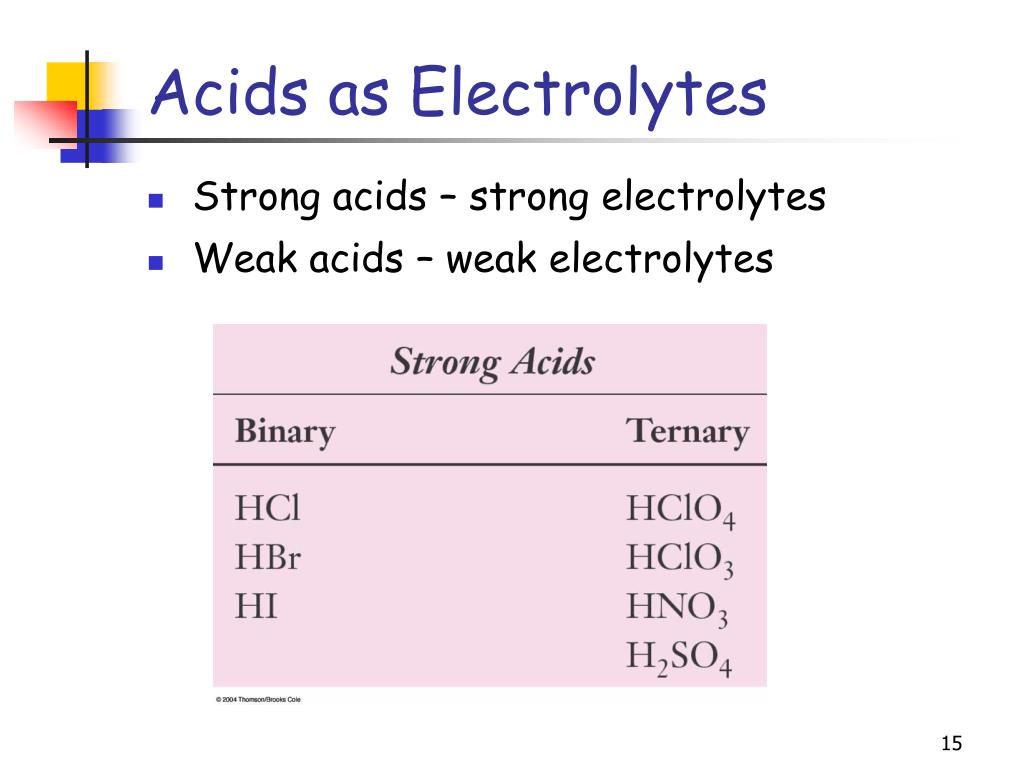
Why Are Strong Acids Also Strong Electrolytes

Okay, let's talk about something that sounds super science-y but is actually kind of funny. We're diving into the world of acids. Not the kind that give you heartburn (though, let's be honest, some of them feel like it). We're talking about strong acids. And their secret life as strong electrolytes. Sounds complicated, right? But stick with me, it's more like a quirky friendship.
Imagine a party. A really, really good party. The kind where everyone knows everyone. That’s kind of like a strong acid when it hits water. It doesn’t just dip its toes in; it cannonballs. And it breaks up into all its little pieces, ready to mingle. These pieces are called ions. Think of them as little charged particles, zipping around like excited party guests.
Now, what’s an electrolyte? In simple terms, it’s anything that has these charged particles floating around in a liquid. And what do these charged particles do? They conduct electricity! So, if you have a bunch of charged particles, you’ve got a good conductor. It’s like having a super-efficient dance floor at that party. Everyone’s moving, and the energy is high.
So, why are strong acids such good electrolytes? Because they’re really good at making these charged particles. They don’t hold back. They’re not shy. When you put a strong acid in water, it’s like saying, “Alright everyone, break up! Go have fun!” And they all break up. Every single molecule. No holding back, no awkward pauses. It’s a complete separation. A total emancipation.
Think of it this way. You know how some people are super indecisive? Like, “Should I have the cake? Or maybe the pie? Hmm, I’ll just stand here and stare at both.” Those are like weak acids. They dip their toes in water and are like, “Uh, is it okay if I split up? Maybe? Or maybe not?” They’re hesitant. They’re unsure. They might break up a little, but a lot of them stay together, holding hands, whispering secrets.
But a strong acid? It’s the opposite. It’s the person who walks into the room and immediately starts organizing a game of charades. It’s all in. It’s committed to the split. Acids like hydrochloric acid (that’s HCl, for you chemistry buffs) or sulfuric acid (H2SO4, the heavy hitter) are prime examples. They don’t just release one hydrogen ion; they release all of them. Every. Single. One. It’s a full-blown, enthusiastic dissociation.
And because they’re so enthusiastic about splitting into charged particles, they become fantastic conductors of electricity. That’s why they’re called strong electrolytes. They’ve got tons of these little charged ions swimming around, ready to carry an electrical current. It’s like they’re made for it. They’re the rock stars of the electrolyte world. They don’t just play a few notes; they give you a full stadium concert of ions.
It’s almost unfair, really. While weak acids are nervously deciding whether to share their toys, strong acids are already out there, building an electric highway. They’re not playing games. They are what they are, and they’re proud of it. They’re not trying to be subtle. They’re making a statement, one charged ion at a time.
So, the next time you hear about a strong acid, just picture that enthusiastic party guest, or the all-in musician. They’re not just strong in their acidity; they’re strong in their ability to break apart and conduct electricity. It’s a powerful combo. A powerful, and dare I say, slightly show-offy, combo. They’re the ones who get the party started and keep the music playing. And honestly, sometimes you just have to admire that kind of commitment, even if it sounds a bit… intense.
It’s like they have a superpower. A superpower for electricity conduction. And it all comes down to their personality. Their willingness to be completely, unequivocally, broken up into charged bits. No regrets. No second thoughts. Just pure, unadulterated ion-generating power. They’re the unsung heroes of the electrical conductivity world, and frankly, I think they deserve a bit more recognition for their dramatic flair.

So, while some might find it a bit much, the strong acid's complete dedication to dissociation is precisely what makes it a stellar electrolyte. It’s not complicated chemistry; it’s just a very determined chemical personality. And that, my friends, is something we can all understand. The enthusiastically broken-apart kind of success.
