Which Electron Configuration Denotes An Atom In Its Ground State
Ever found yourself staring at a jumble of numbers and letters, trying to make sense of it all? Well, you're not alone! Figuring out electron configurations might sound like something reserved for a super-nerdy science lab, but understanding where electrons hang out in an atom is actually a fascinating puzzle that has real-world implications. Think of it like knowing how all the pieces of a complex machine fit together – it helps us understand how that machine works.
So, what's the big deal with electron configurations? Essentially, they tell us the exact arrangement of electrons orbiting the nucleus of an atom. This seemingly small detail is the key to understanding why elements behave the way they do. It's the secret sauce behind chemical reactions, the reason why water is wet, and why your phone screen works. Understanding ground state electron configurations helps us predict how atoms will interact, leading to the development of new materials, medicines, and technologies that improve our lives every single day.
Think about it: the very fabric of our existence is built upon the predictable behavior of atoms. When scientists are designing a new catalyst for cleaner energy, developing a stronger alloy for an airplane, or even creating a new type of fertilizer to grow more food, they're relying on a deep understanding of electron configurations. It’s the fundamental language of chemistry, and mastering it allows us to manipulate matter for our benefit.
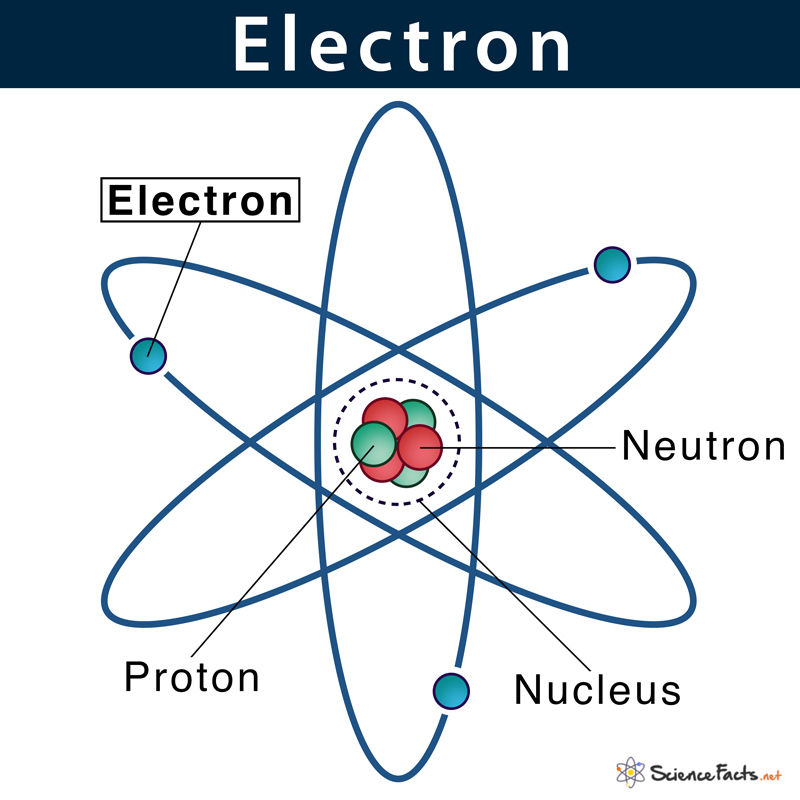
The question, "Which electron configuration denotes an atom in its ground state?" is the central puzzle. The ground state simply means the atom is in its most stable, lowest energy configuration. Electrons, like us, prefer to be in the most comfortable spot possible! They fill up orbitals (think of these as electron "neighborhoods") in a specific order, starting with the lowest energy levels. There are established rules, like the Aufbau principle, Hund's rule, and the Pauli exclusion principle, that dictate this arrangement. When an electron configuration follows these rules, and the electrons are settled in their lowest possible energy levels, you've found the ground state!
So, how can you get a handle on this? Start by familiarizing yourself with the periodic table. It's literally organized based on electron configurations! The blocks on the periodic table (s, p, d, f) correspond to the types of orbitals being filled. Practice writing out the configurations for a few common elements. You’ll quickly see a pattern emerge.
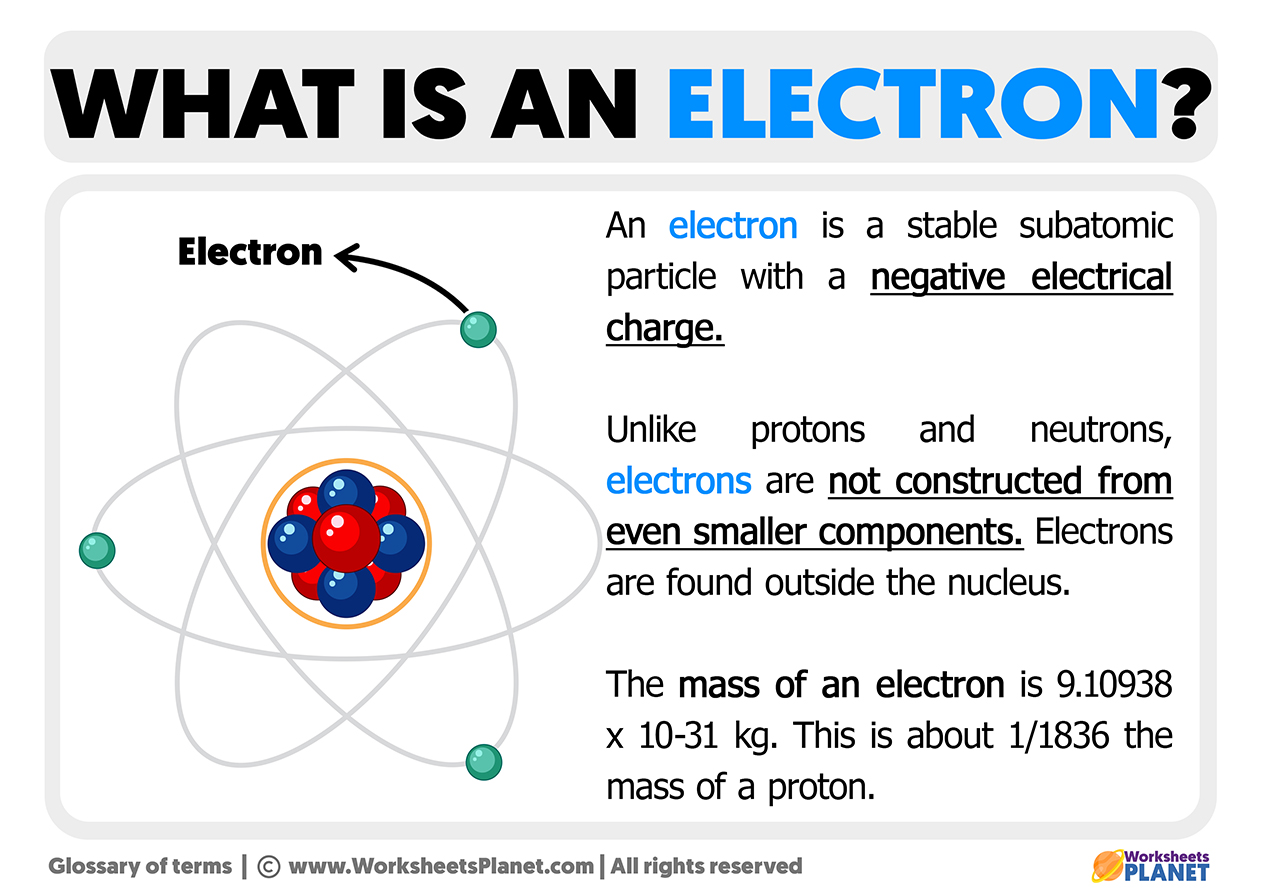
Another great tip is to use visual aids. Many online resources offer interactive tools that let you build atoms and see their electron configurations. Don't be afraid to make mistakes; it’s part of the learning process. Think of it as a fun brain teaser. The more you practice, the more intuitive it becomes, and the more you’ll appreciate the elegant order hidden within the atomic world. It's a skill that unlocks a deeper understanding of the universe around you!
