What Is The Molecular Shape Of No2
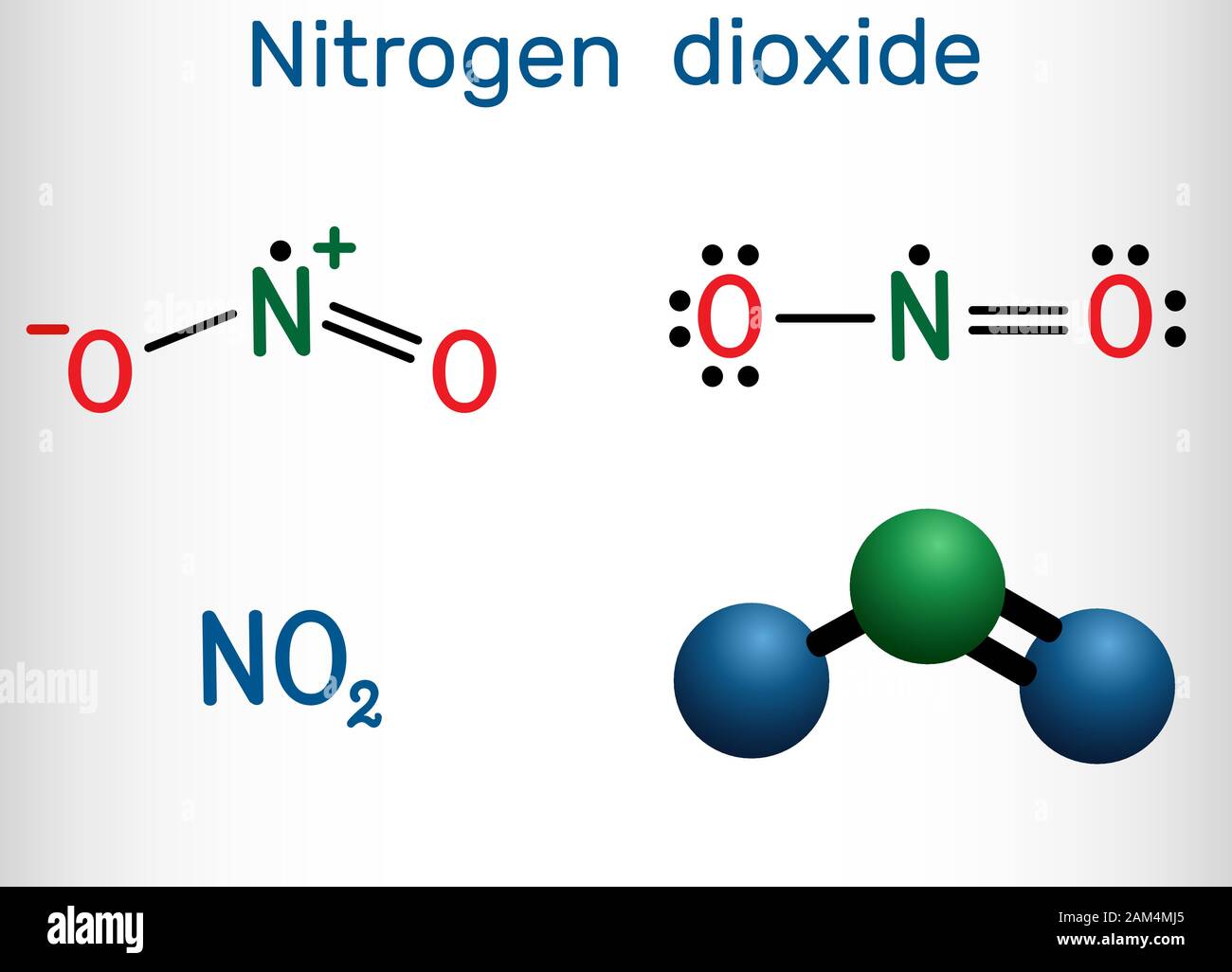
Have you ever been captivated by the intricate beauty of the natural world, particularly the way molecules dance and form? While we might not often think about it, the very structure of these tiny building blocks can inspire some truly fascinating creative endeavors. Today, we're diving into the world of
Now, you might be asking, "What's so special about the shape of NO2?" Well, its bent, V-like structure is not just scientifically significant; it’s a visual marvel that can spark a wealth of artistic expression. For artists, hobbyists, and even curious casual learners, understanding and visualizing this shape can be an incredibly enriching experience.
Imagine the benefits! For an artist, knowing the distinct bent geometry of NO2 can inform their abstract pieces, perhaps translating the molecular forces into dynamic lines and angles. Hobbyists might find it a unique challenge for 3D modeling or even intricate sculpture. And for those simply learning about chemistry, grasping this visual representation makes the abstract concepts of valence electrons and electron domains far more tangible and memorable.
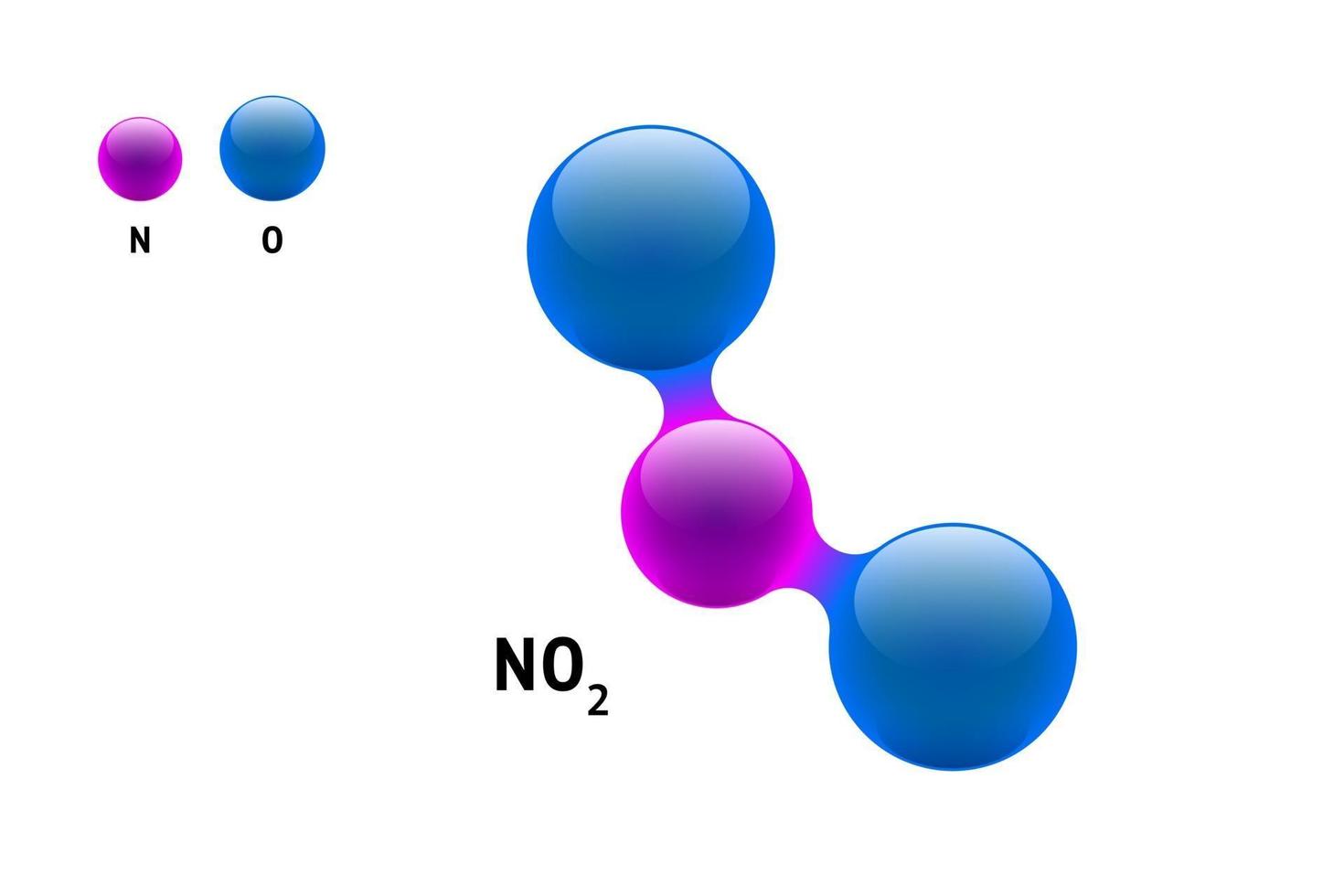
The applications are as varied as your imagination! You could see NO2’s shape inspiring delicate, geometric jewelry designs, or perhaps as a motif in a stained-glass window, playing with light and color through its angular form. Some might even use it as a starting point for abstract painting, focusing on the asymmetry and the potential for movement within the molecule. Think bold strokes representing the bonds, or soft washes of color hinting at the electron cloud.
Thinking of giving it a try at home? It's easier than you might think! Grab some modeling clay or even pipe cleaners and colored balls to represent the atoms. Experiment with different angles until you achieve that characteristic bent shape. You can even find plenty of online resources with diagrams and 3D models to guide you. Don't be afraid to simplify; the goal is to capture the essence of its structure.
Another fun approach is sketching. Start with a central atom and two surrounding ones, then play with the angles. Focus on the

What makes exploring NO2’s molecular shape so enjoyable is its beautiful blend of science and art. It’s a reminder that even the smallest parts of our universe possess an inherent elegance and structure. It's a gateway to understanding, a catalyst for creativity, and a delightful little puzzle to unravel. So, why not embrace the charm of this bent little molecule and see where it takes you?
