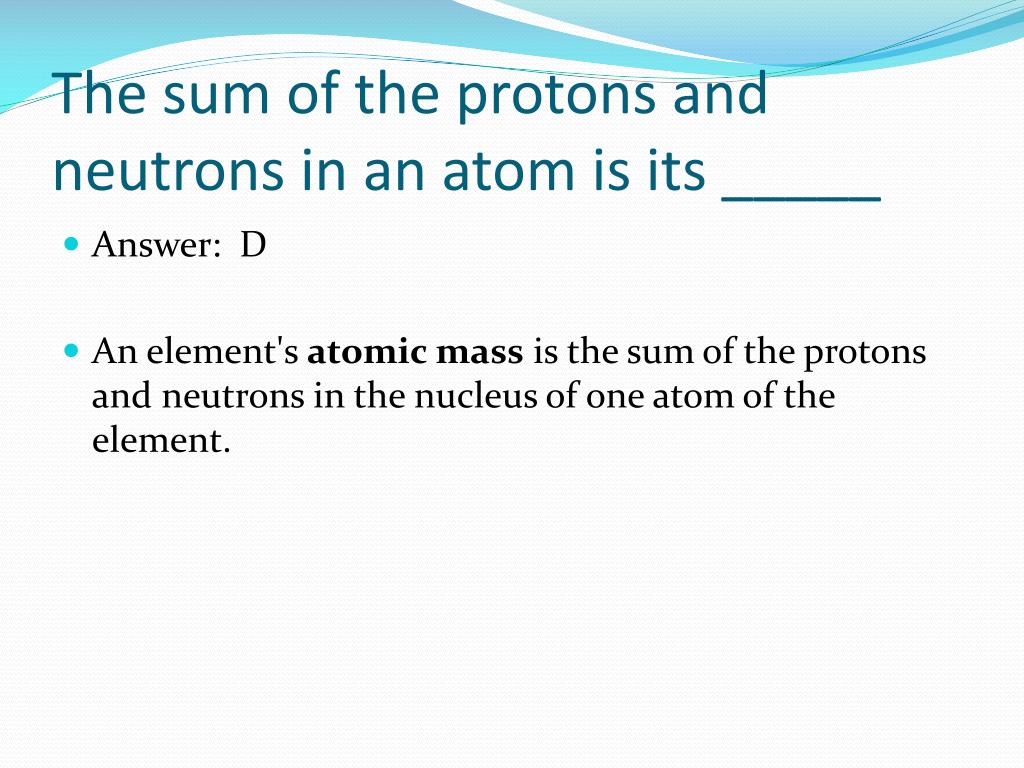
The Sum Of An Atom's Protons And Neutrons

Alright, gather 'round, science enthusiasts and curious cats alike! We're about to dive into something that might sound a tad… well, atom-y. But trust me, it's way cooler and way more important than you might think. Forget those dusty textbooks; we're going to talk about the secret sauce, the powerhouse duo, the ultimate dynamic team that makes up the heart of every single atom. We're talking about the sum of an atom's protons and neutrons!
Now, I know what you're thinking. "Protons? Neutrons? Sounds like something a superhero might shout before a giant laser beam." And you're not entirely wrong! These little fellas are the unsung heroes of the universe, the building blocks of everything. Imagine the universe as a gigantic LEGO set. Atoms are the individual bricks, right? But what makes one brick different from another? What gives it its unique personality and its ability to bond with other bricks in mind-boggling ways? It's all thanks to the stuff crammed inside its teeny-tiny core, also known as the nucleus.
Think of the nucleus as the atom's bustling city center. It's where all the important decisions are made, where the real action happens. And at the heart of this city, ruling the roost, are our stars: the protons and the neutrons.
THE ATOMIC STRUCTURE OF MATTER - ppt descargar
Let's break it down, shall we? First up, we have the protons. These guys are the rock stars of the nucleus. They're like the energetic, always-positive performers who bring the crowd to their feet. They carry a little bit of a spark, a positive charge that says, "Hey, I'm here and I'm ready to go!" The number of protons an atom has is its unique ID card. It's what defines it as a particular element. For example, if an atom has just one proton, BAM! It's a hydrogen atom. If it's got eight protons, that's our friendly neighborhood oxygen, the stuff we breathe and that makes our toast so delightfully crispy.
Then, we have the neutrons. Now, these guys are the cool, calm, and collected support crew. They're like the steady bass guitar in the band, providing the solid rhythm and keeping everything grounded. Neutrons don't have any charge – they're neutral, hence the name! They're basically there to hang out with the protons, giving them a bit of extra heft and keeping that energetic bunch from getting too excited and flying apart. Imagine trying to pack a bunch of super enthusiastic puppies into a tiny basket. You might need some extra stuffing to keep them all contained, right? That's kind of what neutrons do for protons. They add bulk and stability!
So, the sum of an atom's protons and neutrons? That's where the magic really happens! This combined number is like the total weight of the atom's core, its heft, its sheer presence. Scientists have a fancy word for this: mass number. And oh boy, does this mass number tell a story! It's like knowing how much your favorite superhero weighs – it gives you a sense of their power and their potential. A bigger mass number means a heftier atom, a more substantial brick in our cosmic LEGO set.
Let's get a little silly with this. Imagine you're at a pizza party. The protons are the enthusiastic friends who are grabbing the most toppings and talking the loudest. The neutrons are the chill friends who are just happy to be there, maybe passing the napkins. The mass number is like the total number of slices of pizza on the table that everyone's eyeing. More slices mean a bigger pizza, more to go around, and probably a more epic party! An atom with a high mass number is like a generously topped pizza, a real feast for the atomic world. It’s got that extra substance, that satisfying density.
Why is this sum so important, you ask? Well, it dictates so much! It influences how heavy an atom is, how it interacts with other atoms, and even how it behaves in different situations. Think about it: a tiny, light atom is going to act very differently from a giant, heavy one, just like a little nibble of a cracker is different from a whole slice of decadent chocolate cake. The mass number is a key ingredient in the recipe of matter.
And here’s the really cool part: even though we can’t see them, even though they’re smaller than tiny dust bunnies on the moon, these protons and neutrons are constantly working together, holding atoms together, and ultimately, building the entire universe around us. From the air you’re breathing to the screen you’re looking at, it’s all thanks to these incredible, tiny components. So next time you think about atoms, give a little nod to the dynamic duo in the nucleus: the energetic protons and the stable neutrons. Their combined might, their glorious mass number, is the unsung hero behind all of existence!
It’s like the universe is throwing a party, and the protons and neutrons are the ultimate party planners, making sure everything is just right. They're the quiet achievers, the bedrock of reality. So, feel good knowing that even in the smallest of things, there's an incredible amount of power and organization at play. It's a beautiful, intricate dance, and the sum of protons and neutrons is the conductor's baton, keeping the symphony of the universe in perfect harmony!

+is+the+sum+of+the+number+of+protons+and+the+number+of+neutrons+in+the+nucleus+of+the+atom..jpg)