The Major Portion Of An Atom's Mass Consists Of
Hey there, science explorers! Ever wondered what makes up, well, everything? You know, the chair you're sitting on, that sneaky slice of pizza you're eyeing, even the air you're breathing (and yes, we're definitely talking about the stuff that makes up the air, not just your awesome personality)? It all comes down to tiny, tiny things called atoms. Think of them as the ultimate LEGO bricks of the universe. And today, we're going to have a blast peeking inside these miniature marvels to find out where all the oomph – the actual weight – of an atom comes from. Get ready for a whirlwind tour, because the answer is surprisingly... hefty!
Imagine you've got a tiny, microscopic watermelon. Not just any watermelon, mind you, but the densest, most super-concentrated watermelon in existence. This isn't the kind you slice and dice; this is the kind that, if it were any heavier, would probably warp spacetime and attract small, bewildered planets. Now, where do you think most of that watermelon's impressive heft comes from? Is it the watery bits? The rind? Nope! It’s packed right into the very, very core, the absolute heart of that tiny, imaginary melon. And guess what? Atoms are kind of like that!
The Heart of the Matter (Literally!)
When we talk about the "major portion" of an atom's mass, we're essentially talking about its nucleus. This is the atom's super-dense, incredibly important central hub. Think of it as the command center, the power plant, and the grumpy old librarian all rolled into one, guarding all the precious weight. It’s where the real action is, and where all the good stuff that makes atoms heavy hangs out.
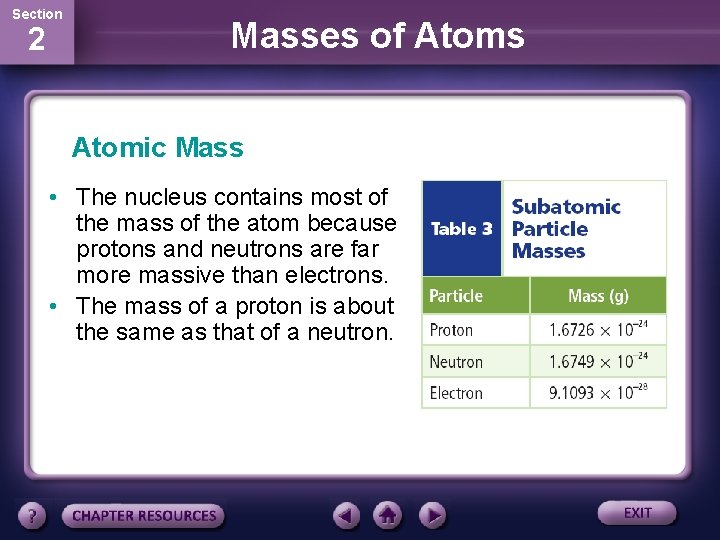
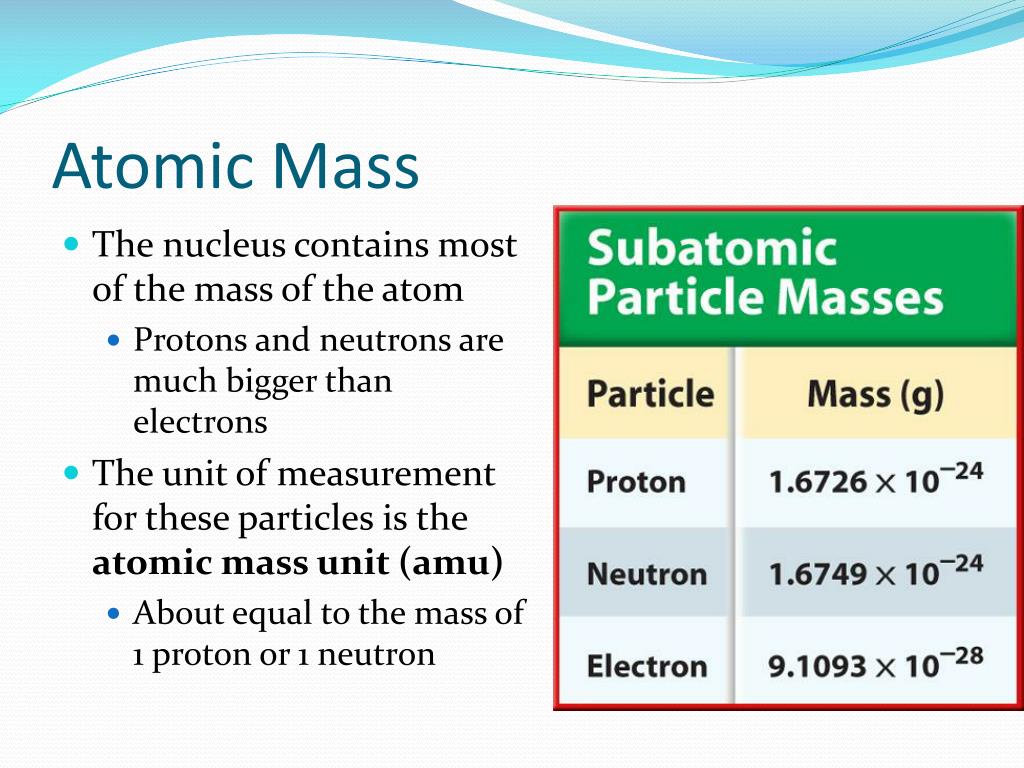
Now, what’s chilling in this atomic VIP lounge? We’re talking about two types of subatomic particles that are the real heavyweights. First up, we have the protons. These guys are like the energetic, positively charged cheerleaders of the nucleus. They’re not messing around; they’re constantly buzzing and contributing a significant chunk of the atom’s weight. Imagine them as tiny, positively charged bowling balls – each one packing a good amount of oomph.
And then, there are the neutrons. These are the calm, cool, and collected ones. They have no charge (hence "neutron" – get it?), but they are absolute chonkers when it comes to mass. If protons are bowling balls, neutrons are like miniature, positively invisible lead weights. They’re the silent anchors, the steady contributors to the atomic weight, making sure everything stays grounded and, well, heavy.
So, picture this: you’ve got a bustling party in the nucleus. The protons are the ones throwing confetti and making noise (positive charge!), and the neutrons are the ones holding up the entire dance floor with their sheer mass, silently ensuring the party doesn't float away into the void. They are the undisputed champions of atomic heft!
What About Those Whizzing Electrons?
Now, you might be thinking, "What about those speedy little electrons that are always zipping around the outside of the atom?" Ah, yes, the electrons! These are the nimble, negative-charged youngsters of the atomic family. They are incredibly important for how atoms interact and form bonds (think of them as the social butterflies of the atom world, making connections!). They're the ones who get all the press, the ones you might picture when you think of an atom.
But here’s the funny part, the little scientific joke: when it comes to mass, these energetic electrons are like the fluff in a giant, delicious marshmallow. They are so incredibly tiny, so ridiculously light, that their contribution to the atom’s overall weight is practically negligible. It’s like trying to weigh down a truck with a single feather. Sure, the feather is there, but it's not going to make a lick of difference to the truck’s scale reading.
So, while the electrons are busy being the life of the party on the outer edges, the real heavy lifting, the major portion of an atom's mass, is happening deep, deep down, in the heart of the nucleus, thanks to those mighty protons and their massively important, if quieter, friends, the neutrons. They are the unsung heroes, the powerhouses, the reason why matter, as we know it, has substance. Without them, our universe would be a lot more… ethereal and a lot less… hefty!
Isn't that cool? The most important part of an atom's weight is tucked away in its incredibly dense core. It's a tiny world packed with colossal importance. So next time you pick something up, feel its weight, just remember the bustling, heavyweight party happening inside every single atom. It’s a microscopic powerhouse, and its mass is all about that incredible nucleus!
