Open System Closed System And Isolated System
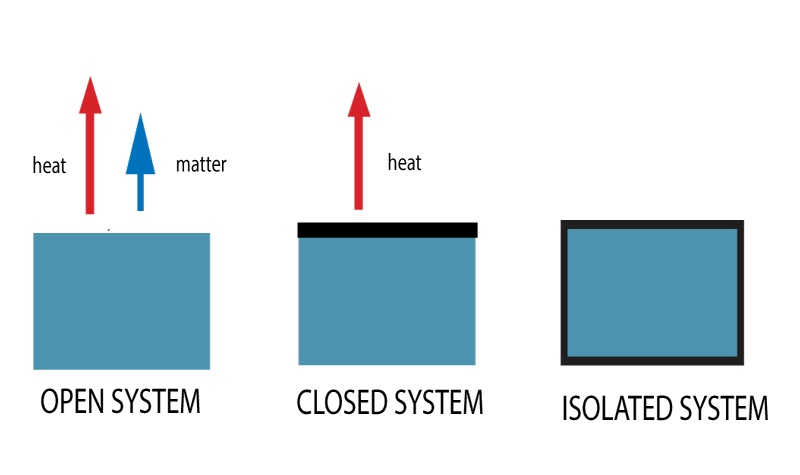
Imagine your favorite comfy blanket. It’s a bit like a closed system. It keeps you warm, but some heat still escapes, and maybe a stray crumb or two can sneak in.
Think about a steaming mug of hot chocolate on a chilly afternoon. That’s a perfect example of an open system. Heat is definitely escaping into the air, and if you’re not careful, a little bit of the outside world (like a fly!) might decide to join your cozy drink.
Now, picture a magical, perfectly sealed thermos. This is where we start to approach the idea of an isolated system. Ideally, nothing gets in or out – no heat, no matter. It’s like a tiny, self-contained universe of deliciousness.
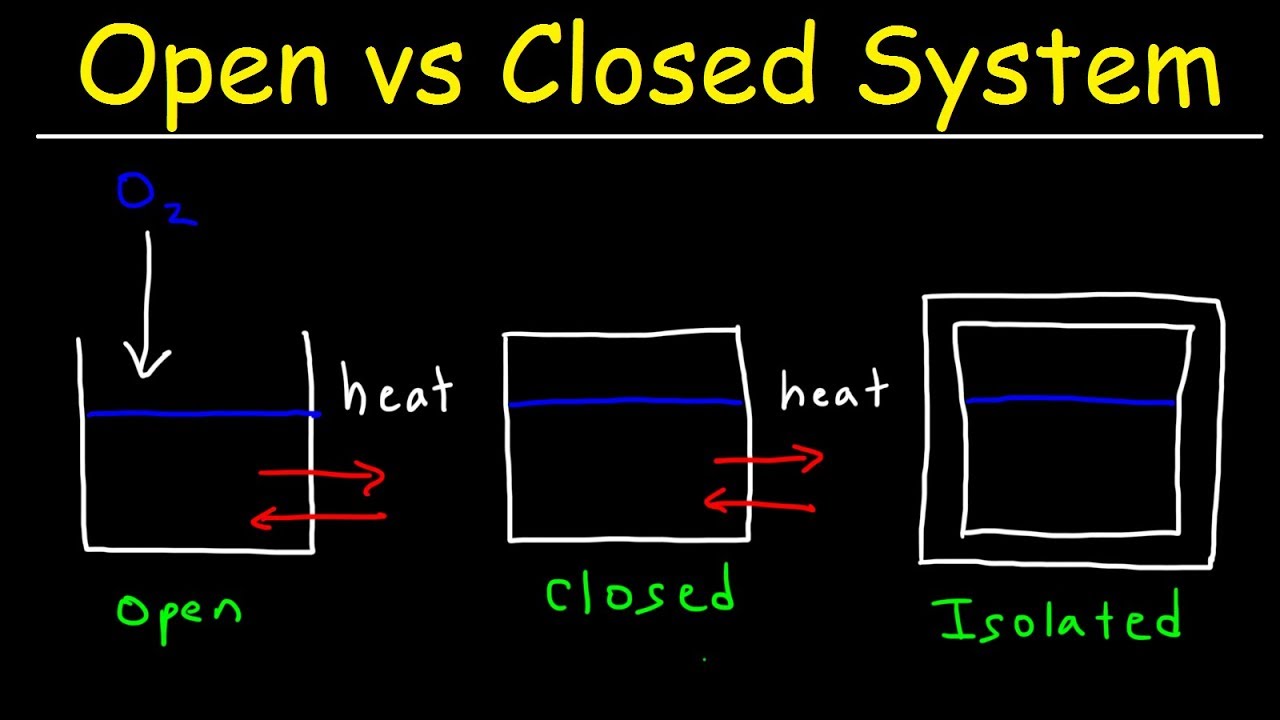
Your Kitchen: A Tiny Universe of Systems
Your kitchen is a bustling hub of activity, and guess what? It’s full of these invisible systems! From the sizzling stove to the frosty fridge, each appliance plays its part.
Let’s start with your trusty old toaster. When you pop in some bread, it's essentially an open system. Heat escapes from the slots, and the crumbs you can never quite get rid of are definitely "matter" making its presence known.
Your oven, when you’re baking cookies, is also a grand open system. The delicious aroma of chocolate chips and sugar wafts out, sharing its joy with the entire house. Heat, of course, is a constant companion to this delicious process.
But what about your refrigerator? It’s striving to be a bit more like a closed system. It works hard to keep the cold air in and the warm air out, trying to maintain its internal chill.
Still, even the best refrigerator isn't perfectly sealed. Every time you open the door, it’s a momentary breach, allowing a quick exchange with the outside. It’s a tiny, chilly negotiation.
The Great Outdoors: Nature’s Systems at Play
Step outside, and you’re immersed in the grandest open system imaginable: Earth itself! Our planet is constantly exchanging energy and matter with the vastness of space.
Think of a sunbathing lizard. It’s actively absorbing solar energy (heat) and also losing some heat to the surrounding air – a classic open system. It’s a direct interaction with its environment.
A rainstorm is another beautiful illustration of an open system. Water falls from the sky, and the air around it is warmed by the sun and cooled by the rain. It's a dynamic, ever-changing dance.
Even a sealed bottle of water left out in the sun shows us aspects of these systems. While the bottle tries to keep things contained, heat from the sun still warms the water inside, making it a bit of an open system to energy.
When Things Get Serious (and a Little Sci-Fi)
Now, let’s talk about the elusive isolated system. In the real world, truly isolated systems are incredibly rare, like finding a unicorn or a perfectly brewed cup of coffee that stays hot forever.
The universe, as a whole, is often considered the ultimate potential isolated system. However, scientists are still debating whether that's truly the case! It's a cosmic mystery.
Scientists in labs often work hard to create near isolated systems. They use special vacuum chambers and super-insulating materials to minimize energy and matter exchange. It's like building a tiny, perfect bubble.
These experiments are crucial for understanding fundamental laws of physics. It’s like trying to hear a whisper in a silent room – you need to block out all the other noise to hear it clearly.
From Your Lunchbox to the Stars
Let’s bring it back to something you love: your lunchbox! A well-packed lunchbox with an ice pack is trying its best to be a closed system for a few hours. It keeps your sandwich cool and your juice from getting warm.
But eventually, the ice melts, and heat creeps in. It’s a noble effort, but nature always finds a way to nudge things towards balance. It’s a gentle reminder that even our best efforts at containment are temporary.
Think about your favorite board game. When you’re all engrossed in playing, for that moment, your little table might feel like a closed system. The outside world fades away, and it's just you and the game.
But then someone’s phone rings, or a pet jumps on your lap. Suddenly, your game is interacting with the outside, reminding you it’s an open system after all. It’s a bit like life – always a little bit of an exchange happening.
The concept of systems helps us understand how things work, from the smallest atom to the grandest galaxy. It’s a way of categorizing the world and seeing the invisible boundaries (or lack thereof) between things.
So, the next time you enjoy a warm cup of tea or feel the sun on your face, take a moment to appreciate the fascinating dance of open, closed, and the ever-elusive isolated systems all around you. It’s a beautiful, interconnected universe!
Even a hug is a sort of temporary open system! You share warmth and maybe a few giggles, but eventually, you have to let go. It’s a lovely, fleeting exchange of energy and connection.
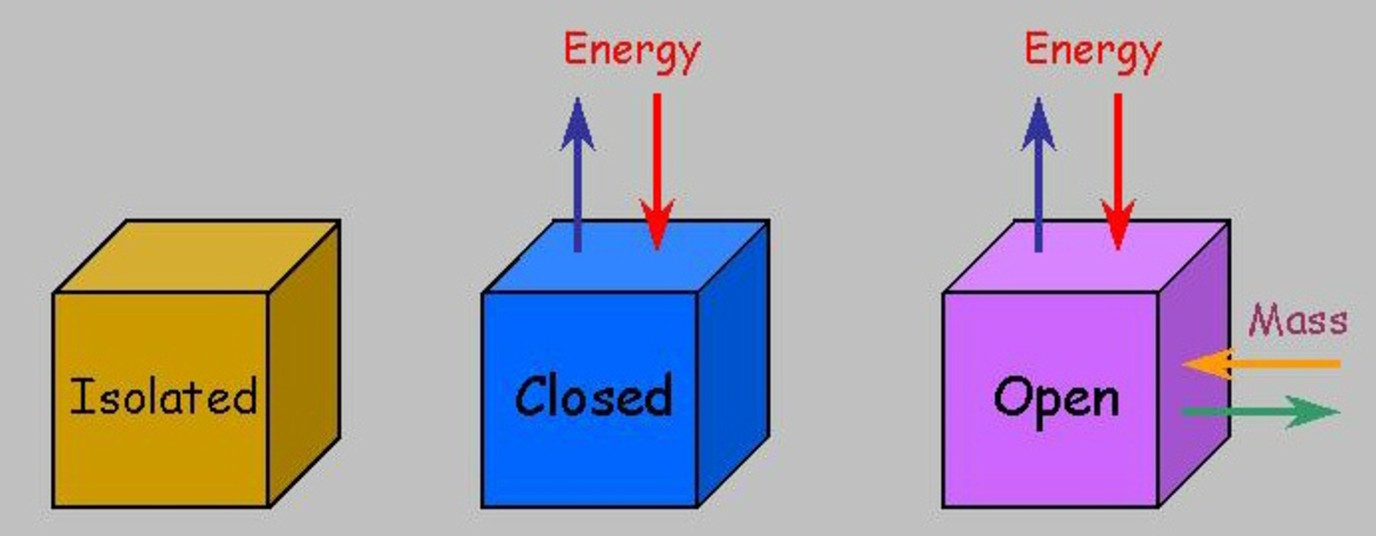
The world is a constant symphony of energy and matter moving, exchanging, and transforming. Understanding these simple ideas – open, closed, and isolated – is like getting a secret decoder ring for how everything in the universe is connected. It's surprisingly fun!
