Is A Peptide Bond A Covalent Bond

Alright, let's dive into the nitty-gritty of what makes us, well, us. We're talking about the tiny building blocks of life, the proteins, and the special handshake that holds them together. You've probably heard the term "peptide bond" floating around, maybe in a science class or a documentary about, like, super-muscular athletes. But what is it, really? And, to get to the heart of it, is a peptide bond a covalent bond? Stick with me, because we're going to break this down like a perfectly baked cookie, with just the right amount of crumble and gooeyness.
Think about your favorite LEGO set. You've got all these individual bricks, right? Each brick is cool on its own, but the real magic happens when you snap them together to build something awesome – a spaceship, a castle, or that ridiculously complex Millennium Falcon. In the world of biology, those LEGO bricks are called amino acids. They're the fundamental units, the individual pieces. And to build those amazing protein structures, like the ones that make your muscles work or help you digest that burrito, these amino acids need to link up. And that's where our friend, the peptide bond, comes into play.
So, is it covalent? Let's get to the punchline early: Yes, a peptide bond is indeed a covalent bond. Ta-da! Mind. Blown. Okay, maybe not mind-blown, but hopefully, a little spark of understanding has ignited. Now, let's unpack why that's the case, without making your brain feel like it just ran a marathon.
Imagine you're at a potluck. Everyone brings a dish. Now, imagine you want to share your amazing macaroni and cheese with your neighbor, who brought their legendary seven-layer dip. You can't just hold your dish out and hope it magically connects. You need a way to physically link your food to theirs, even if it's just for a moment of sharing. In the biological world, this "linking" is more permanent and fundamental. It's a chemical connection.
A covalent bond, in its simplest terms, is like two people deciding to share their toys instead of just passing them back and forth. They're not just borrowing; they're literally holding onto the same toy together. In chemistry, this "sharing" happens with electrons. Atoms have electrons, which are like tiny, energetic little particles buzzing around. When atoms form a covalent bond, they essentially agree to share some of their electrons. This sharing creates a strong, stable connection between them.
Think of it like a really good friendship. You and your bestie might share a Netflix password, or maybe even a favorite hoodie. It's a shared resource, a connection that's stronger than a casual acquaintance. Covalent bonds are that level of commitment for atoms. They're sharing electrons, and that sharing is what holds molecules together.
Now, let's bring it back to amino acids. Each amino acid has a specific structure, but they all have a few key parts. For our discussion, the important bits are something called an amino group and a carboxyl group. These are like the specific connection points on our LEGO bricks. When two amino acids decide to buddy up and form a chain (a polypeptide, which is basically a fancy word for a protein precursor), their amino group and carboxyl group get together.
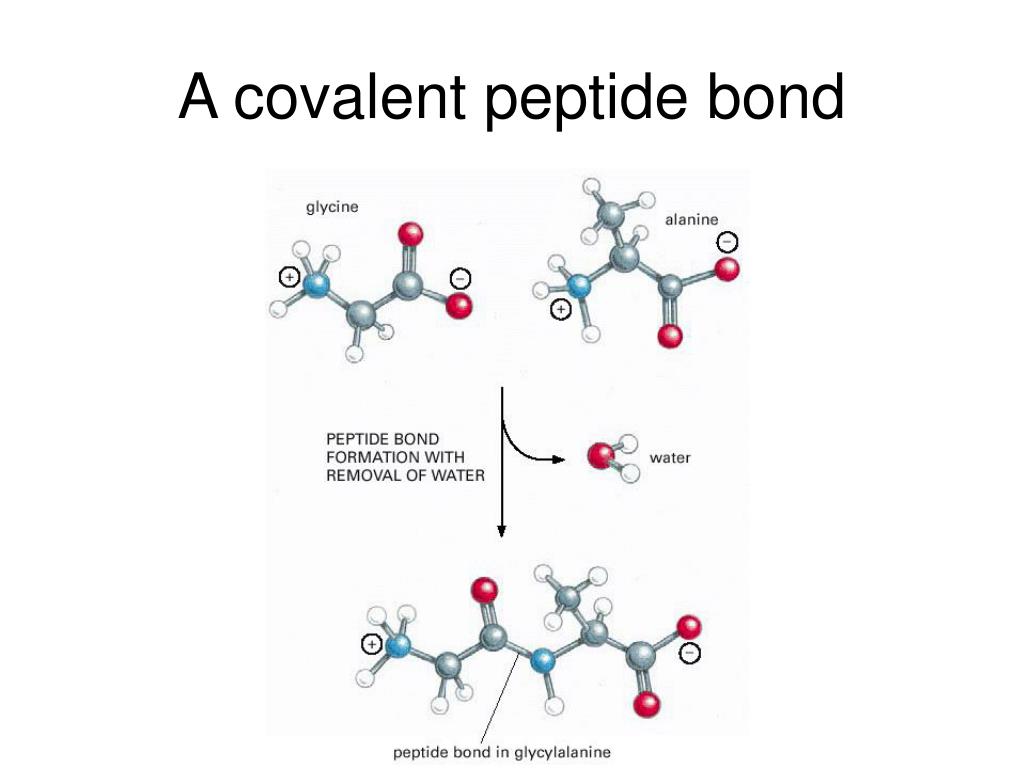
It's a bit like a chemical wedding. The carboxyl group of one amino acid, let's call it "Amy," has a part that's looking to ditch a hydrogen atom. And the amino group of the next amino acid, "Barry," is ready to welcome that hydrogen and its attached oxygen. So, Amy's carboxyl group says, "Hey Barry, I've got this OH group I'm willing to part with, and Barry, you've got an H just begging for a new home!" And Barry, being amenable, says, "Sure thing, Amy! Let's do this!" In this union, they get rid of a water molecule (H₂O – like a little biological divorce where the water molecule is the alimony). And what's left? A strong, stable bond connecting Amy and Barry, allowing them to become part of a longer protein chain.
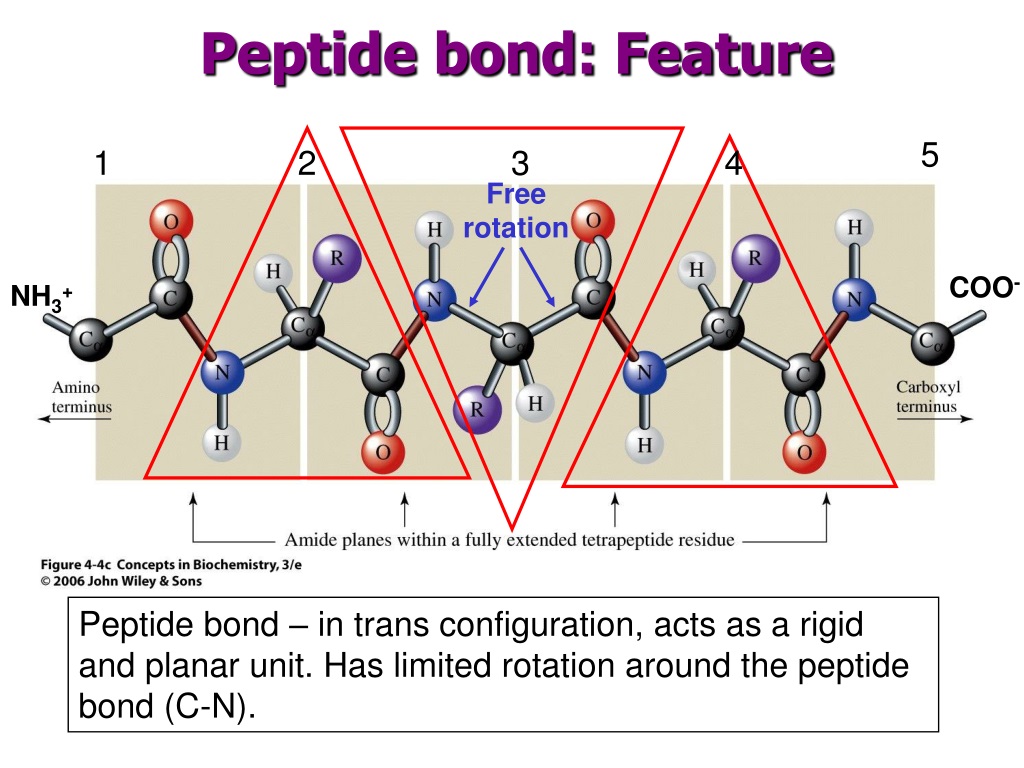
This new connection? That's the peptide bond. And because it's formed by the sharing of electrons between atoms (specifically, the carbon of the carboxyl group and the nitrogen of the amino group), it's a prime example of a covalent bond. It’s not just a weak attraction, like when you really like the smell of your neighbor's baking cookies. This is a fundamental, sharing-of-electrons, "we're in this together" kind of connection.
Why is this so important? Well, imagine building that LEGO spaceship with only sticky tape holding the bricks together. It would fall apart faster than a politician's promise. Proteins need to be robust. They need to withstand the hustle and bustle of the cell. The strength of the covalent peptide bond ensures that these protein chains can maintain their structure and perform their vital functions. It's the chemical equivalent of superglue, but way cooler and way more natural.
Think about how your hair grows. It's made of a protein called keratin. Keratin strands are long chains of amino acids linked by peptide bonds. If those bonds were weak, your hair would be as flimsy as a wet noodle. The sturdy covalent nature of the peptide bond is what gives your hair its strength and structure. It's also what allows for things like muscle contraction, enzyme activity, and even the signals that travel through your nerves. All thanks to these robust little covalent connections.
So, when you hear "peptide bond," just picture those amino acids, our biological LEGO bricks, linking up in a permanent, electron-sharing embrace. It's a fundamental part of how life is built, molecule by molecule. It's a covalent bond, plain and simple, and it's responsible for a heck of a lot of what makes us tick.
It’s like when you’re making a friendship bracelet. You don’t just loosely loop the threads. You tie knots, right? Those knots are strong and secure. A peptide bond is like a super-strong, chemically engineered knot that permanently links two amino acids. And when you have a whole bunch of these knots tied in a row, you get a long string – the beginnings of a protein. It’s a beautiful, intricate process that’s happening inside you right now, without you even having to lift a finger.
It’s easy to get lost in the jargon, isn’t it? "Peptide bond," "covalent bond," "amino acid." It sounds like something out of a sci-fi movie. But at its core, it's just about things sticking together in a way that makes sense for building something bigger and more complex. Like how you and your significant other might share a bank account – it’s a shared resource, a commitment, a bond that’s stronger than just saying "I like your shirt."
The formation of a peptide bond is a classic example of a dehydration reaction. Fancy term, I know. But it just means that in the process of forming the bond, a water molecule is released. Think of it like this: you’re trying to get two friends to hold hands. To do that, they might need to let go of something they were holding onto, like a drink. That drink is the water molecule, and once it’s gone, their hands are free to interlock. And that interlocking? That’s your covalent peptide bond.
So, to reiterate for those who might be zoning out (no judgment, I do it too!): A peptide bond is a covalent bond. It’s the chemical link that forms when the carboxyl group of one amino acid reacts with the amino group of another. This sharing of electrons creates a stable, strong bond, essential for the formation of proteins. It’s the foundation of so much of what makes life work, from the microscopic to the macroscopic.
Next time you’re marveling at a beautiful muscle, or enjoying a meal that your body is diligently processing, take a moment to appreciate the humble peptide bond. It’s the unsung hero, the silent workhorse, the covalent connector that holds the intricate tapestry of life together. And that, my friends, is pretty darn cool. So, yeah, it's covalent. Solid as a rock, or at least, solid as a very well-built protein.
Remember that feeling when you finally get that stubborn knot out of your shoelace? It takes some effort, and a bit of manipulation, but once it's out, the shoe is secure. Peptide bond formation is a bit like that, but on a molecular level. It’s a specific chemical interaction that results in a secure connection, ready to withstand the forces it will encounter. It’s not a fleeting handshake; it’s a full-on, electron-sharing embrace that defines the backbone of life itself.
