How To Know Charges Of Transition Metals

Alright, gather 'round, you lovely humans and possibly some curious squirrels who've learned to read! We're diving headfirst into the wild, wacky world of transition metals. Now, these aren't your garden-variety, predictable elements. Oh no. These guys are the rebels, the rockstars, the ones who can't quite make up their minds about how many electrons they want to show off. Think of them as the teenagers of the periodic table, constantly changing their "charge" depending on who they're hanging out with.
So, how do we figure out what kind of charge these fickle friends are rocking? It's like being a detective, but instead of a trench coat and a magnifying glass, you've got a bit of chemistry know-how and a whole lot of patience. And maybe a good cup of coffee, because, let's be honest, some of these ions are more confusing than trying to assemble IKEA furniture without the instructions.
The Periodic Table: Your Not-So-Secret Weapon
First things first, let's talk about the periodic table. It's not just a fancy poster for your science classroom; it's a cheat sheet! While the main group elements (you know, the ones in groups 1 and 2 and the last few columns) are pretty straightforward – they generally stick to one or two charges like a toddler sticks to a juice box – the transition metals, those colorful blocks in the middle, are a different story. They’re the moody artists of the element world, capable of expressing themselves in multiple ways.
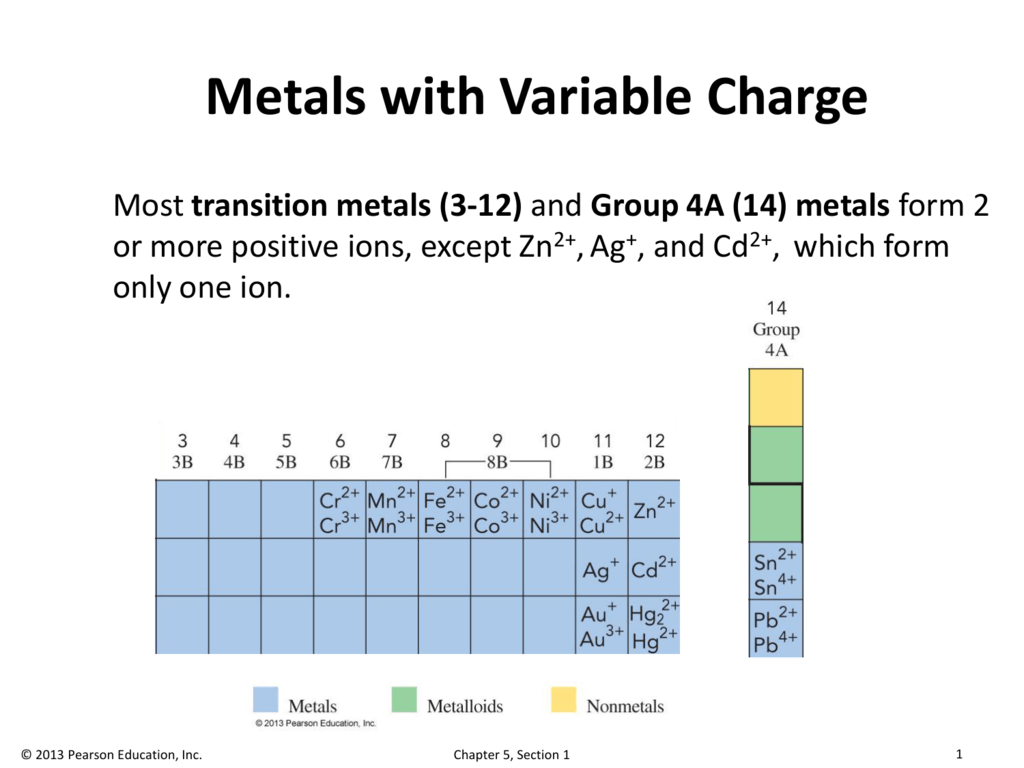
Think of it this way: Sodium (Na) is like your super reliable, always-on-time friend. They're almost always going to be +1. Easy peasy. But Iron (Fe)? Iron can be +2 or +3. It’s like, "Do I feel like being a mild-mannered iron today, or a fierce, powerful ferric ion? Decisions, decisions!" It’s enough to make your head spin faster than a carnival ride.
The "Usual Suspects" (But Not Really)
Now, even with their unpredictable nature, there are some trends. It's like knowing your friend Gary will always bring the questionable dip to the party, even if you don't know what that dip is made of. For transition metals, a lot of them prefer to be in a lower, more common charge state. For example, iron often likes being Fe²⁺ (ferrous) or Fe³⁺ (ferric). Copper can be Cu⁺ or Cu²⁺. Manganese, bless its heart, can rock a whole spectrum from +2 all the way up to +7. That's like wearing seven different hats in one day!
But here’s the kicker: these are just preferences. They're not commandments. They're more like suggestions from the element elders, which the transition metals often ignore with a mischievous twinkle in their electron shells. So, relying solely on what's "usual" is like planning your vacation based on a weather forecast from last year. It might be a good starting point, but don't be surprised by the unexpected shower (or, in this case, a different charge).
Context is King (or Queen, or Non-Binary Monarch)
The real secret to figuring out a transition metal's charge is to look at its chemical friends. Who are they bonded to? What's the overall vibe of the compound? This is where the detective work really kicks in.
Let's take a classic example: iron oxide. You might see it written as FeO or Fe₂O₃. Now, oxygen is a notoriously predictable element. It loves to be -2. It's like the overachiever of anions, always seeking that perfect electron configuration. So, if we know oxygen is -2, we can work backward.
In FeO, you've got one iron and one oxygen. The whole thing is neutral (meaning no overall charge). If oxygen is -2, then for the compound to be neutral, iron has to be +2. Simple, right? It's like balancing a seesaw. One side goes down, the other has to go up the same amount.
But then you look at Fe₂O₃. Now we have two iron atoms and three oxygen atoms. Oxygen is still doing its -2 thing. So, three oxygens give us a total negative charge of 3 * (-2) = -6. To make the whole thing neutral, the two iron atoms together need to have a total positive charge of +6. That means each individual iron atom must be +3. See? The same metal, just hanging out with a different number of oxygen buddies, and poof – a different charge!
Polyatomic Ions: The Wild Cards
Now, things get a little more interesting when you throw in polyatomic ions. These are like little gangs of atoms that stick together and carry a charge as a unit. Common culprits include sulfate (SO₄²⁻), nitrate (NO₃⁻), and phosphate (PO₄³⁻). These guys are like the established rulers of their own little kingdoms, and you have to respect their charges.
Let's say you see something like copper(II) sulfate, CuSO₄. We know sulfate (SO₄) is a tough cookie with a -2 charge. Since the overall compound is neutral, the copper must be +2 to balance out that -2 sulfate. Easy! But what if you saw copper(I) chloride, CuCl? Chlorine, like oxygen, is usually pretty straightforward, hanging out at -1. So, for CuCl to be neutral, copper has to be +1.
It's like playing a game of "who owes whom what." You figure out the known charges, and the transition metal is the one left to make things add up to zero. Sometimes, they’re generous and give away more electrons (positive charge), and sometimes they’re a bit more stingy (lower positive charge).
The Nomenclature Clue: It's in the Name!
And here's a little secret weapon for your arsenal: the name of the compound itself! If you see something like "iron(II) chloride," that little Roman numeral (II) is your direct clue. It's telling you, in no uncertain terms, that the iron in this particular compound is rocking a +2 charge. It's like the compound is wearing a name tag that says, "Hello, my iron is +2!"
This is a lifesaver, especially in introductory chemistry. They literally spell it out for you! If it's "iron(III) chloride," then that iron is +3. If it's "manganese(VII) oxide," you know that manganese is showing off its +7 electron-donating skills. It’s like the compound is shouting its charge from the rooftops. Marvelous!
The "Less Common" Charges (For the Adventurous)
Now, for the truly brave souls, you should know that transition metals can sometimes sport even weirder charges, especially in more complex compounds or coordination complexes. Think of scandium (Sc), which is almost always +3. It's the reliable one in the transition metal family. But then you have others that can dip into really high oxidation states, like tungsten or osmium. They can be +6, +7, even +8! It’s like they’ve had a few too many espressos and are just overflowing with electron-giving energy.
But don't let those extreme examples scare you off. For most everyday chemistry you'll encounter, sticking to the context clues – the other elements, the polyatomic ions, and especially those handy Roman numerals – will get you 99% of the way there. The other 1%? That's where the real fun, and maybe a few late nights with textbooks, begin.
So, next time you encounter a transition metal, don't panic! Just put on your detective hat, have a sip of your beverage, and remember: it’s all about who they’re with and what the overall story is. These metal ions are just trying to find their place in the chemical crowd, and sometimes, that means trying on a few different charges. And that, my friends, is the beautiful, chaotic, and often entertaining truth about transition metals!
