How To Determine Strong And Weak Electrolytes

Alright, gather 'round, you magnificent science enthusiasts and curious cats! Today, we're diving into the electrifying world of electrolytes. No, no, don't worry, we're not talking about rocket science here, just some super cool, everyday stuff that makes things work. Think of it like this: some drinks are like a party with a super-powered DJ, while others are more like a quiet afternoon with a librarian. We're going to learn how to spot the difference!
So, what in the name of all that's fizzy are electrolytes? Imagine tiny little charged particles, like microscopic bouncy balls with a positive (+) or negative (-) charge, zipping around in a liquid. These little guys are the VIPs when it comes to conducting electricity. And when we talk about electrolytes, we're usually talking about stuff dissolved in water, because water is like the super-highway for these charged particles.
Now, how do we figure out who's a strong electrolyte and who's a weak electrolyte? It all boils down to how much of a splash they make in the water. Strong electrolytes are like the rockstars of the electrolyte world. When you toss them into water, they go completely bonkers and break apart into a gazillion charged particles. They're all-in, 100% dissociated! Think of them as those friends who show up to a party and instantly know everyone, turning the volume up to eleven!
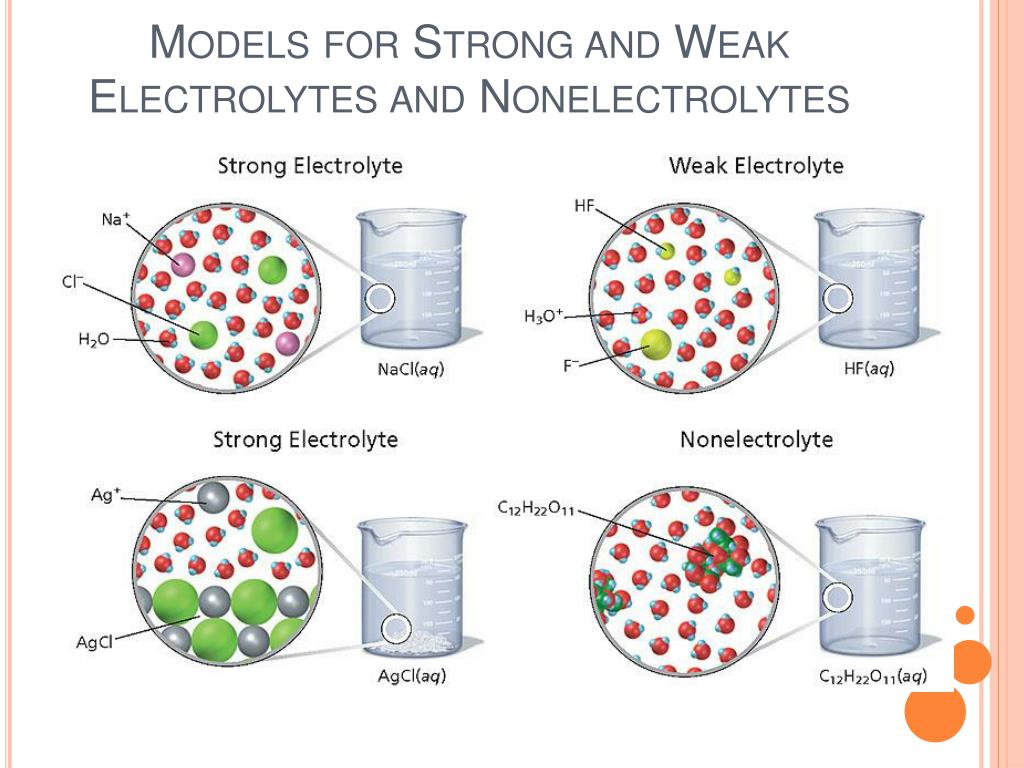
The champions of strong electrolytes are usually salts, strong acids, and strong bases. Let's take a common salt, like the stuff you sprinkle on your fries: sodium chloride (NaCl). When you dissolve table salt in water, it doesn't just sit there looking pretty. Nope! It splits into positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). They're everywhere, like confetti after a parade! This means a solution of sodium chloride is an excellent conductor of electricity. It's practically buzzing with energy!
Another superstar strong electrolyte is something like hydrochloric acid (HCl). When this acid hits the water, it completely dissociates into hydrogen ions (H+) and chloride ions (Cl-). Bam! Instant conductivity. And for bases, think of sodium hydroxide (NaOH). It's another one that can't resist breaking apart into sodium ions (Na+) and hydroxide ions (OH-) in water, ready to conduct electricity like a champ.
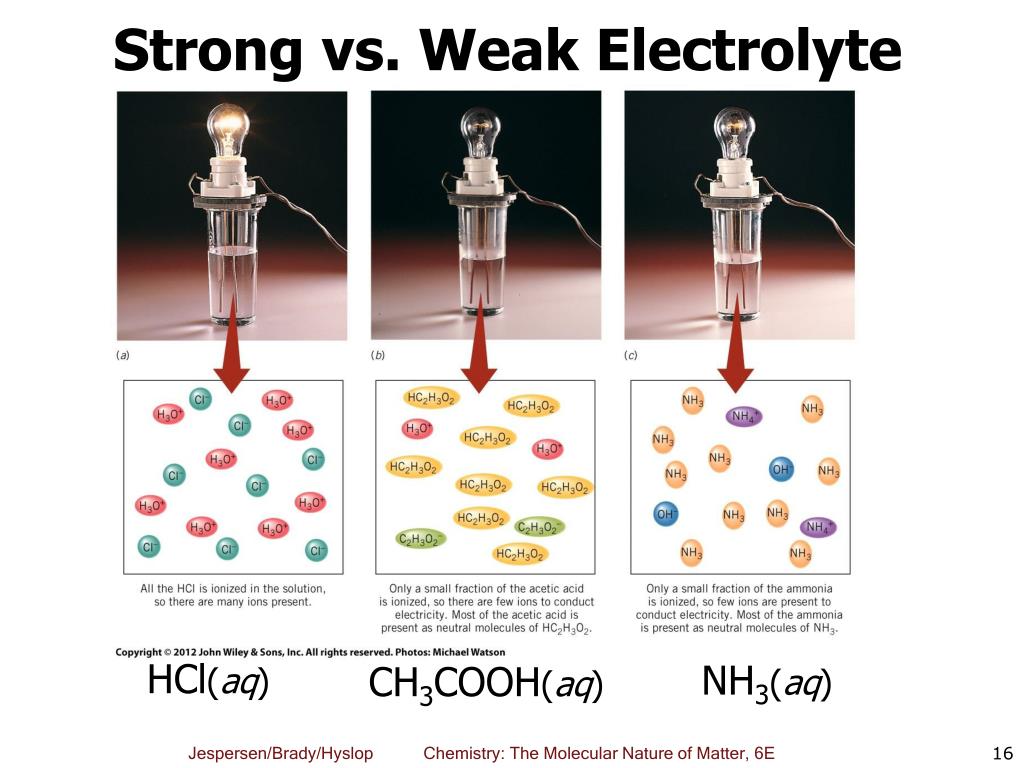
Imagine you're trying to power a tiny light bulb. If you use a strong electrolyte solution, it's like plugging it into a mega-watt power plant. That bulb is going to shine bright like a diamond! If you use something else... well, let's just say the bulb might just flicker sadly, or not light up at all.
On the other hand, we have the chill cousins: weak electrolytes. These guys are more reserved. When you put them in water, only a small fraction of them decide to break apart into charged particles. Most of them prefer to hang out together, like a group of friends at a low-key gathering. They're more like, "Eh, maybe a few of us will split, but the rest of us are good."
Weak electrolytes are typically weak acids and weak bases. Take acetic acid, the stuff that makes vinegar sour. When you put acetic acid (CH3COOH) in water, only a little bit of it breaks into hydrogen ions (H+) and acetate ions (CH3COO-). The majority of the acetic acid molecules are still intact. So, a vinegar solution isn't going to conduct electricity as well as a salt solution. It's like trying to start a rave with just a ukulele instead of a full band.
Another example is ammonia (NH3). When ammonia dissolves in water, it forms ammonium ions (NH4+) and hydroxide ions (OH-), but only to a limited extent. It's not a full dissociation party. So, an ammonia solution is a much weaker conductor of electricity compared to a strong base like sodium hydroxide.
So, how do you actually tell the difference in real life? Well, one of the easiest ways is to look at the label! If it says it's a strong acid, strong base, or a common salt (like NaCl, KCl, MgSO4), it's probably a strong electrolyte. If it says weak acid or weak base, you've got yourself a weak electrolyte.
Another awesome trick is to use a conductivity meter. These handy gadgets measure how well a solution conducts electricity. A solution with strong electrolytes will make the meter go wild, showing a high conductivity reading. A solution with weak electrolytes will give a much lower reading. It's like a report card for your electrolytes!
Think of it as a spectrum of enthusiasm. Strong electrolytes are shouting from the rooftops, "We are here and we are charged!" Weak electrolytes are whispering, "Just a few of us are feeling it today." And then there are things that don't break apart at all, like sugar (sucrose) or alcohol (ethanol). These are called non-electrolytes. They're the party poopers of the electrical conductivity world. They dissolve, sure, but they keep their charges all to themselves, like secret agents. They don't conduct electricity one bit. So, if you're looking to conduct electricity, stick to the strong and maybe a little bit of the weak, but leave the sugar for your tea!
It's a simple concept, but incredibly important in everything from how our bodies work to how batteries function. So next time you're sipping on something, take a moment to ponder: is it a party animal electrolyte, or a chill vibe electrolyte? You're now officially an electrolyte detective!
