Determine The Oxidation State Of Carbon In Co
Hey there, science explorers and curiosity champions! Today, we're diving into a super-duper exciting topic that might sound a little intimidating at first, but trust me, it's as easy as pie and way more fun than doing your laundry! We're going to unravel the mystery behind the oxidation state of carbon in CO. Yep, that’s right, we’re talking about carbon, the superstar element that’s in all of us, in our food, and even in that fizzy drink you might be sipping right now!
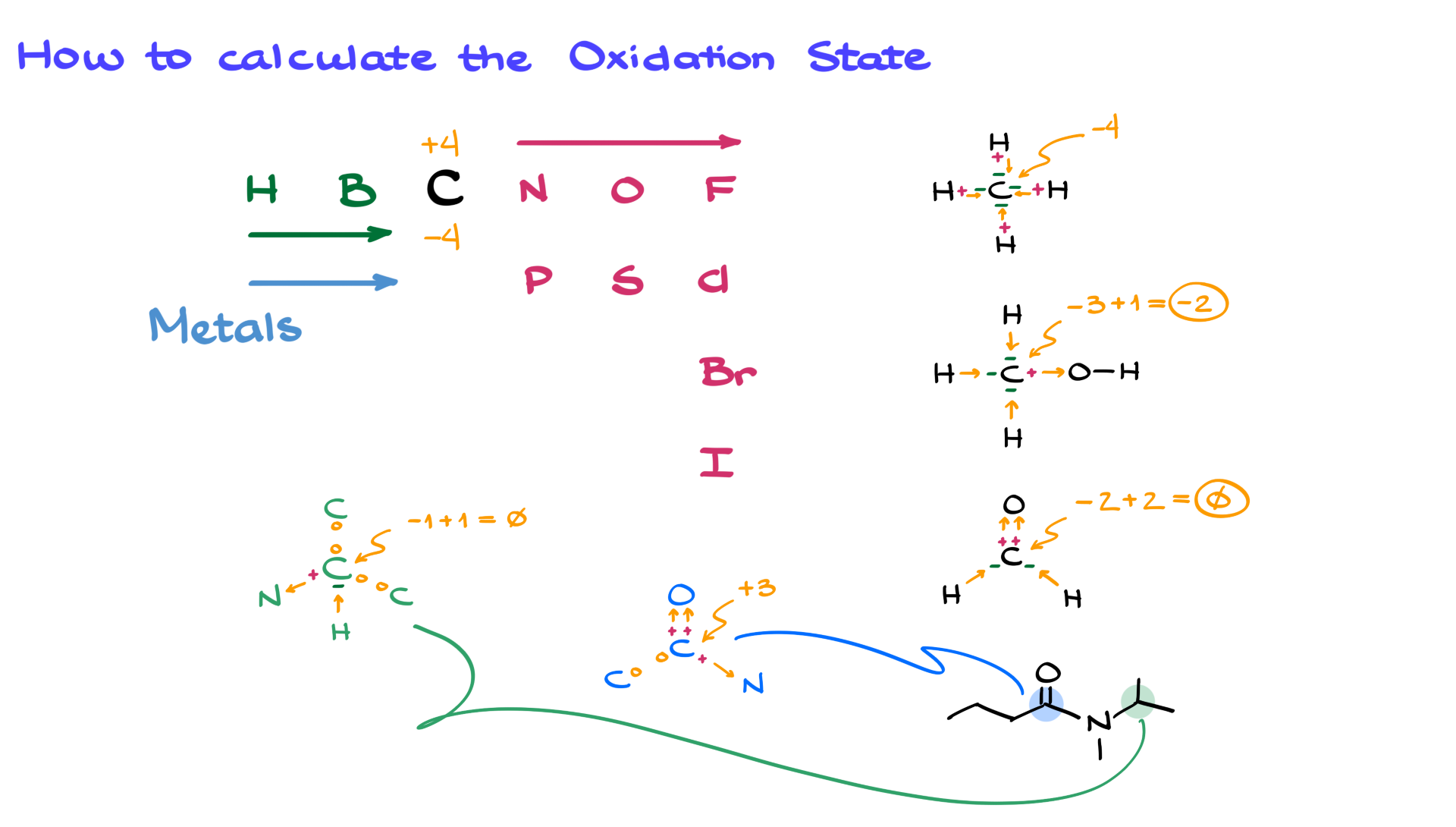
Now, you might be wondering, "What in the world is an 'oxidation state'?" Think of it like carbon's imaginary "score" in a chemical game. This score tells us how many electrons it's either gained or lost when it hangs out with other atoms. It’s like a little badge it wears, showing off its electron-sharing or electron-stealing prowess!
Our main character today is CO, which is also known as carbon monoxide. Don't let the "monoxide" part scare you; it's just a fancy way of saying "one oxygen." This is a pretty important molecule, and understanding its carbon's oxidation state is like cracking a secret code that helps us understand how it behaves. It’s a tiny molecule with a big personality!
So, how do we figure out carbon's score in CO? It's like playing detective! We need to know the "scores" of the other players in the game. In CO, we have carbon and oxygen. We need a little bit of a cheat sheet here, a chemical rulebook if you will.
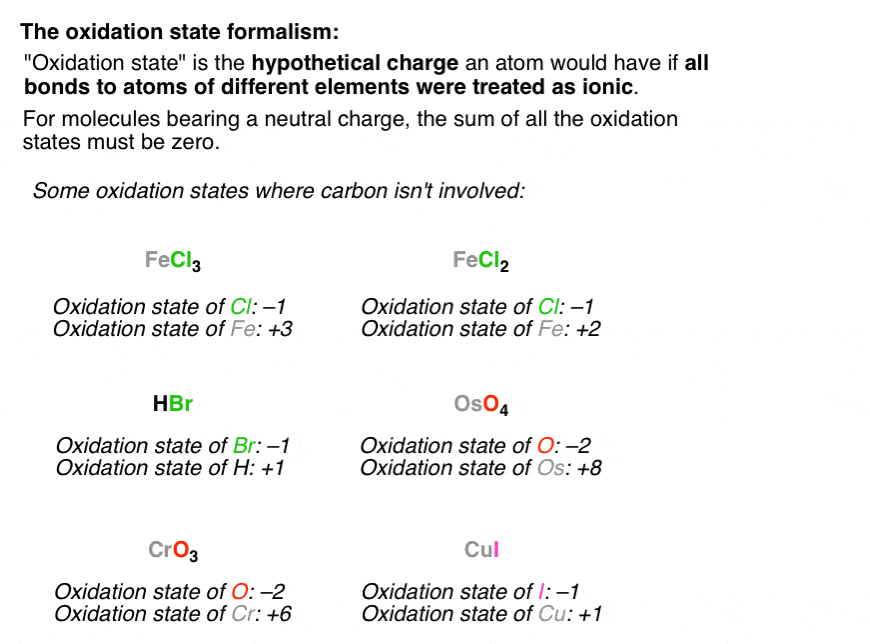
One of the golden rules of chemistry, and a super helpful one for our game, is that oxygen almost always gets a score of -2. Think of oxygen as the super popular kid in class who always gets to borrow your favorite pen – it likes to take electrons! So, in CO, oxygen is happily holding onto two electrons, giving it a score of -2.
Now, let's bring in our star, carbon. We know that in a neutral molecule like CO, the total score of all the atoms must add up to zero. It's like a balanced seesaw – everything needs to be level! So, if oxygen has a score of -2, what does carbon need to be to make the whole thing add up to zero?
This is where the fun math comes in, and it's not even the boring kind! We have: Carbon's score + Oxygen's score = 0. We know oxygen's score is -2. So, we plug that in: Carbon's score + (-2) = 0.
To get carbon's score, we just need to do a tiny bit of rearranging, like a magician pulling a rabbit out of a hat! We add 2 to both sides of the equation. And voilà! Carbon's score = +2.
So, in the molecule CO, the oxidation state of carbon is a fantastic +2! Isn't that neat? It means that in this partnership, carbon is playing a bit of a generous role, giving up some of its electrons to its buddy oxygen. It's like carbon is saying, "Here, oxygen, you can have these!"
Let's imagine carbon as a really friendly person who loves to share. When it teams up with oxygen in CO, it's like carbon is at a party and oxygen is the one with the really cool cookies. Carbon is so eager to be friends that it basically hands over some of its electron-cookies to oxygen, leaving carbon with a positive vibe – a positive oxidation state!
This +2 score is super important. It tells us how carbon is feeling electron-wise in this specific compound. It’s like knowing if your friend is feeling energized or a bit tired; it affects how they interact with others. Carbon at +2 is ready to mingle and react in certain ways.
Think about it this way: if carbon was a superhero, in CO, it's not the super-villain hoarding all the power. Instead, it's more like the helpful sidekick, lending its energy to make the team stronger. It’s a team player, and that +2 score is its team jersey!
Now, you might have seen carbon in other compounds, and its oxidation state can change! It's like carbon can wear different costumes depending on who it's hanging out with. For instance, in CO2 (carbon dioxide), carbon has a different score. But that’s a story for another day!
For today, we’re focused on the awesome CO and our champion carbon with its +2 oxidation state. It’s a simple calculation, but it unlocks a world of understanding about chemical reactions and how molecules behave. It’s like having the key to a secret chemical clubhouse!
So, the next time you hear about carbon monoxide, you can impress your friends by knowing that the carbon in it is rocking a +2 oxidation state. It’s a little bit of chemistry magic that’s easy to remember. Just think of oxygen taking its usual -2, and carbon happily balancing it out with a +2.
This concept of oxidation states is like the alphabet of chemistry. Once you know it, you can start reading all sorts of chemical sentences and understanding complex chemical stories. It's the foundation for so many amazing discoveries and technologies!
And remember, even though carbon monoxide is a gas that needs to be handled with care in high concentrations, understanding its chemistry, including the oxidation state of its carbon, is a safe and fascinating journey. We're not experimenting with anything dangerous here; we're just playing with numbers and elements in our minds!
So, give yourself a pat on the back! You've just learned a fundamental concept in chemistry. You've figured out the oxidation state of carbon in CO. That's like solving a puzzle or mastering a new dance move. High fives all around!
This simple +2 score for carbon in CO is a building block. It helps chemists predict how this molecule will react, what it might form, and how it fits into the grand tapestry of chemical reactions. It’s like knowing the personality of a character in a play; it helps you understand the whole story.
The beauty of it is its simplicity. No need for complex formulas or confusing jargon. Just a few basic rules and a bit of arithmetic, and you’re a chemistry whiz! It’s proof that science can be accessible and even, dare I say, delightful!
So, let’s celebrate this small but mighty piece of knowledge. The oxidation state of carbon in CO is +2. It's a testament to the elegant order that exists in the universe, even at the atomic level. And you, my friend, have just decoded a little bit of that magic!

Keep that curiosity alive, and remember that every element has a story to tell, and understanding their oxidation states is like reading the first chapter of those exciting tales. You’ve got this!
