An Atom Is Best Described As
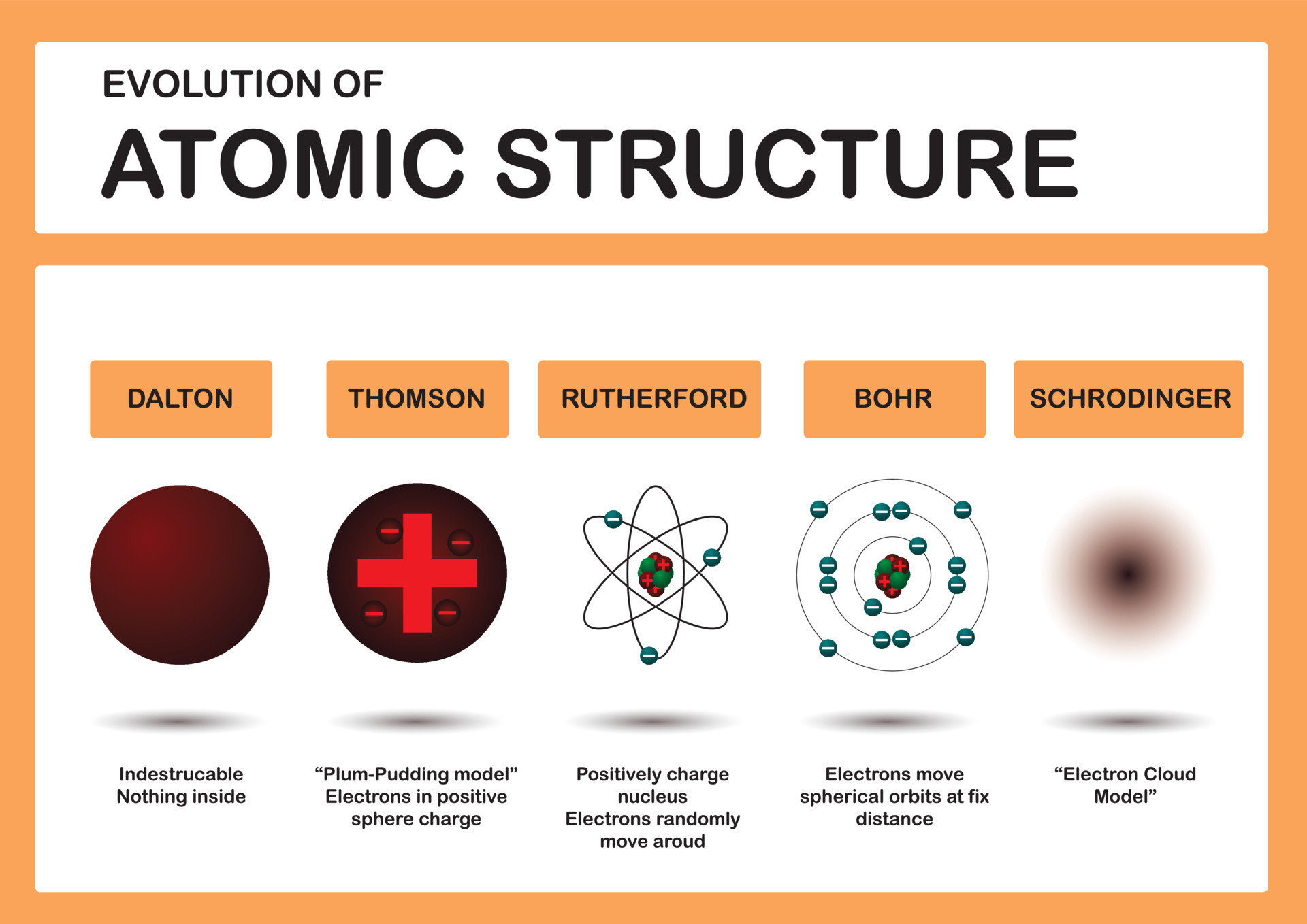
So, what exactly is an atom? Imagine you're a tiny, tiny explorer, so small you could fit on the tip of a dust mote. Even then, you'd still be way too big to see an atom. They're the ultimate LEGO bricks of the universe, the teeny-tiny building blocks that make up absolutely everything around you. Seriously, EVERYTHING! That comfy chair you're sitting on? Made of atoms. That delicious cookie you're dreaming of? Atoms. Even the air you're breathing, those invisible wisps that keep you alive? Yep, you guessed it – atoms!
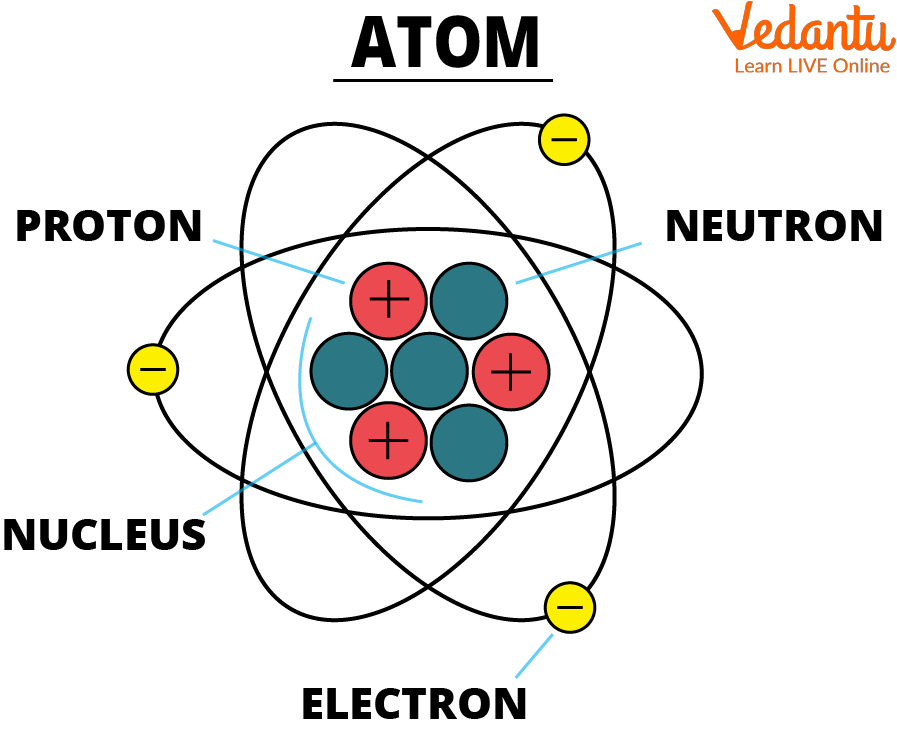
Think of them like those minuscule, almost magical specks of glitter you find stuck to everything after a craft project. Except, instead of sparkly plastic, they're made of even smaller, even stranger things. At the very heart of every atom is a bustling little neighborhood called the nucleus. This nucleus is like the town square, where all the important stuff happens. And what's in this town square? Two kinds of super-tiny particles: protons and neutrons. Protons are like the enthusiastic, slightly loud citizens, always chattering away, and they carry a positive little spark, like a happy little "yes!" in their hearts. Neutrons, on the other hand, are the calm, collected types. They don't have any spark, no positive or negative vibe, they're just… there, chilling and keeping things steady, like a grandparent quietly knitting in the corner.
Now, buzzing around this busy nucleus, like hyperactive toddlers at a birthday party, are these other little characters called electrons. These electrons are tiny, super-speedy, and they carry a negative charge – like a little "nope" or a shy "shhh." They zoom around the nucleus in what we like to call orbits, but it's not like planets going around the sun. It's more like a swarm of super-fast gnats dancing around a lamp post, but in a very, very organized way. They don't just wander aimlessly; they're drawn to the positive protons in the nucleus like magnets, but they're also zipping around so fast they don't just crash into them. It’s a delicate dance, a cosmic ballet happening at speeds we can only dream of!

The number of protons in the nucleus is like the atom's name tag. It's what makes an atom unique. If an atom has one proton, it's a hydrogen atom. If it has two protons, it's a helium atom (the stuff that makes balloons float so funnily!). If it has eight protons, it's an oxygen atom – vital for breathing and for making those delightful bubbles in your bath! Every element on the periodic table, from the mighty iron in your cutlery to the exotic gold in jewelry, has a specific number of protons that defines it. It’s like having a secret code for each and every kind of stuff in the universe!
Think of atoms as the ultimate multitaskers. They're incredibly small, incredibly fast, and incredibly important. Without them, there would be no stars, no planets, no pizza, and definitely no us!
Sometimes, atoms like to team up. They hold hands, or rather, they share their electrons. This is how molecules are born! A molecule is just a group of atoms stuck together, like best friends holding hands. Water, for instance, is a molecule made of two hydrogen atoms and one oxygen atom, all holding hands very tightly. It’s the ultimate atomic friendship circle! This sharing and bonding is what creates all the different substances we encounter every single day. It’s the reason why a diamond is hard and shiny, while a balloon filled with helium is light and floaty. It all comes down to how these tiny little atom buddies decide to hang out together.
And here’s the truly mind-boggling part: atoms are mostly empty space! Yes, you read that right. The nucleus is super tiny, and the electrons are zipping around in a vast emptiness. If you could somehow "squish" all the empty space out of all the atoms in your body, you'd be smaller than a tiny grain of sand. It’s a bit like looking at a vast starry night sky – you see all these points of light, but the darkness in between is immense. Atoms are like tiny, dense star systems, with a super-bright, super-small sun (the nucleus) and incredibly fast, incredibly distant planets (the electrons) orbiting in a massive void.
So, next time you look around, remember that everything you see, touch, and feel is a dazzling, dynamic dance of atoms. They're the silent, invisible engineers of reality, the cosmic dust bunnies that have somehow managed to build a universe. They are the fundamental "yes" to existence, the tiny sparks of energy that come together to create the grand spectacle of life and matter. They're pretty amazing, if you ask me!
